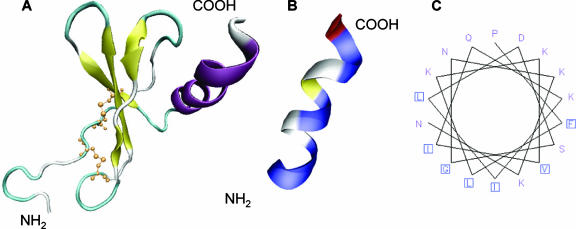
FIG. 2.
Predicted structure of GCP-2/CXCL6 in relation to antibacterial activity. (A) Predictive model based on known structures of other members of the chemokine family. The NH2 terminus, most likely, is an unstructured region followed by three antiparallel β-sheets that are held together by disulfide bonds involving the cysteines of the CXC motif (yellow). The COOH terminus is presumably an α-helix (purple). (B) The putative COOH-terminal α-helix (amino acids 56 to 77) has an amphipathic structure where hydrophobic (blue) and hydrophilic (white/yellow) amino acids are arranged in a polarized fashion. (C) The amphipathic character of the COOH terminus is further elucidated by depicting it as a helical wheel, giving a view of a helix from a protein sequence, looking down the axis of the helix.