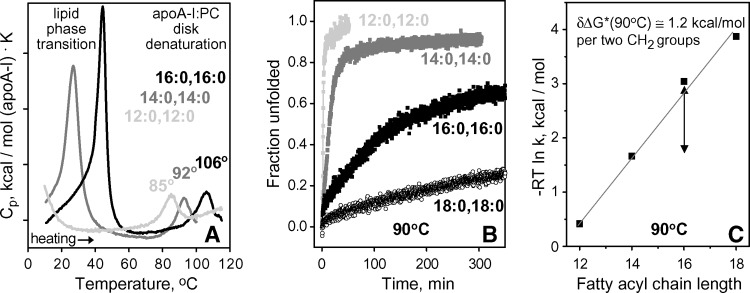
Fig. 5.
Chain length effects on heat denaturation of apoA-I complexes with fully saturated diacyl PCs. A: Partial specific heat capacity Cp(T) recorded upon heating of discoidal apoA-I complexes with DLPC, DMPC, or DPPC at a rate of 90°C/h. Low-temperature peaks are centered near Tc of the gel-to-liquid crystal lipid phase transition (Table 1), and high-temperature peaks are centered at the apparent temperature Tm,app of the disk denaturation. Increase in Tm,app with chain length increase suggests disk stabilization. B: Time course of protein unfolding upon disk denaturation at 90°C monitored in T-jumps by CD at 222 nm. Slower unfolding in complexes with longer-chain PCs indicates higher kinetic stability of these complexes. The unfolding rates k(90°C), which were determined from single-exponential fitting of the Θ222(t) data, were used to obtain the plot in C. C: Kinetic disk stability ΔG*(90°C) ∼ −RT ln k(90°C) as a function of chain length. The slope of the plot corresponds to disk stabilization by δΔG*(90°C) = 1.2 kcal/mol upon chain length increase by two CH2 groups (shown by double arrow).