Abstract
Apolipoprotein A-V (apoA-V) is an important regulator of plasma levels of triglyceride (TG) in mice. In humans, APOA5 genetic variation is associated with TG in several populations. In this study, we determined the effects of the p.185Gly>Cys (c.553G>T; rs2075291) polymorphism on plasma TG levels in subjects of Chinese ancestry living in the United States and in a group of non-Chinese Asian ancestry. The frequency of the less common cysteine allele was 4-fold higher (15.1% vs. 3.7%) in Chinese high-TG subjects compared with a low-TG group (Chi-square = 20.2; P < 0.0001), corresponding with a 4.45 times higher risk of hypertriglyceridemia (95% confidence interval, 2.18–9.07; P < 0.001). These results were replicated in the non-Chinese Asians. Heterozygosity was associated, in the high-TG group, with a doubling of TG (P < 0.001), mainly VLDL TG (P = 0.014). All eleven TT homozygotes had severe hypertriglyceridemia, with mean TG of 2,292 ± 447 mg/dl. Compared with controls, carriers of the T allele had lower postheparin lipoprotein lipase activity but not hepatic lipase activity. In Asian populations, this common polymorphism can lead to profound adverse effects on lipoprotein profiles, with homozygosity accounting for a significant number of cases of severe hypertriglyceridemia. This specific apoA-V variant has a pronounced effect on TG metabolism, the mechanism of which remains to be elucidated.
Keywords: polymorphism, Chinese Americans, triglycerides, heart disease, lipoprotein lipase, high density lipoprotein, haplotypes, single nucleotide polymorphism
Hypertriglyceridemia, a common disorder in the United States, is now recognized as an independent risk factor for coronary heart disease (CAD) (1–4). It is a significant element in the metabolic syndrome, a constellation of clinical features linking insulin resistance, hyperlipidemia, low HDL cholesterol (HDL-C), diabetes, obesity, and hypertension with CAD.
Unlike the other members of the multigene apolipoprotein family, APOA5 was discovered only recently. The mRNA was upregulated along with that from several novel genes following partial hepatectomy (5), suggesting that it plays a role in the provision of lipid for membranes of newly formed cells. It was also reported as a result of a comparative study of the human and mouse genomes (6). The apolipoprotein A-V (apoA-V) protein is present in plasma chylomicrons, VLDL, and HDL (7). The existence of functional farnesoid X receptor and peroxisome proliferator-activated receptor α response elements in the promoter suggests an important role in hepatic triglyceride (TG) metabolism. When the gene was overexpressed in mice, either as a transgene or by adenovirus transfection, there was a noticeable decrease in the levels of TG (6). ApoA-V knockout mice, in comparison, displayed a 4-fold increase in TG (6).
The gene for apoA-V (APOA5) has a close homology with that for apoA-I (APOA1) and apoA-IV (APOA4). APOA5 lies close to these two genes as well as that for apoC-III (APOC3) in the cluster on chromosome 11q23. Variations at this locus have been known for a long time to be important in influencing the characteristics and levels of lipoproteins. It has particularly been implicated, at least in some kindreds, in the familial combined hyperlipidemia phenotype (8–14). We have shown in three dyslipidemic populations that the rare allele of the single nucleotide polymorphism (SNP) rs662799 (−1131T>C) in the promoter of APOA5 is associated with elevated TG and VLDL cholesterol (VLDL-C) and with lower levels of HDL (15). A number of other studies have also shown a correlation between the −1131T>C variant and plasma TGs (6, 16–23). Its importance with regard to hypertriglyceridemia was recently reinforced in a genome-wide association study (24).
The APOA5 SNP rs2075291 (c.553G>T; p.185Gly>Cys) was associated with hypertriglyceridemia in a Taiwanese population (25). Subsequently, a population with CAD in Nanjing, China, was reported to have a higher frequency of the T allele than a control group (26). Carriers had 64 mg/dl higher TG than noncarriers. We hypothesized that the c.553G>T polymorphism in the APOA5 gene is an important regulator for TG metabolism in individuals of Asian origin. We examined the effect of this SNP in a population of Chinese ancestry living in a Western environment to replicate association studies done in Taiwan and China. In four other Asian groups, we also examined the frequency and impact of this APOA5 SNP. Haplotypes were constructed with 11 APOA5 gene SNPs in the carriers of the c.553T polymorphism to determine the effect of the alleles.
Additional biochemical analyses were performed in subgroups of carriers and noncarriers of the T allele to gain a better understanding of the mechanism underlying the hypertriglyceridemia associated with this polymorphism. We measured plasma levels of apoA-I, apoA-V, apoB, apoC-II, apoC-III, apoE, angiopoietin-like protein 3 (angptl3), lipoprotein-lipid composition, and postheparin LPL and HL activities.
METHODS
Study design
This report is a genetic association study of the APOA5 gene p.185Gly>Cys (rs2075291) SNP (exon 4) among samples from the University of California, San Francisco (UCSF), Genomic Resource in Arteriosclerosis (GRA) (27). Informed consent was obtained for all subjects, and the UCSF Committee of Human Research Internal Review Board approved the study protocol.
All subjects in the GRA of Chinese ancestry living in northern California were selected for the study. Plasma levels of total cholesterol and TG were available for all subjects, and lipoprotein lipid measurements were also available for most subjects. All values were obtained after an overnight fast. None of the subjects was taking lipid-lowering medications. Subjects were dichotomized on the basis of fasting levels of plasma TG above (high TG; n = 152) or below (low TG; n = 148) 150 mg/dl. The National Cholesterol Education Program (28) has defined TG values below 150 mg/dl as normal.
All additional subjects of Asian ancestry in the GRA were screened to establish the SNP frequency in non-Chinese Asian-American populations. In this replication study, high TG (n = 139) and low TG (n = 101) were selected as above.
Of the 541 Asian subjects selected for these two studies, most attended UCSF clinics, and 19% were volunteers who attended health fairs in San Francisco.
Lipid and lipoprotein analysis
Cholesterol and TG contents of plasma and lipoproteins were determined by automated chemical analysis (29). VLDL (d < 1.006 g/ml) was prepared by ultracentrifugation (30). HDL-C was measured after precipitation of apoB-containing lipoproteins with dextran sulfate and magnesium (31). LDL cholesterol (LDL-C) was calculated as TC minus HDL-C plus VLDL-C or using the Friedewald equation when the TG was <400 mg/dl (32). Standards were provided by the Centers for Disease Control.
ELISA was used to determine the plasma contents of apoA-V (23, 33), apoC-II (34), apoC-III (34), apoE (34), and angptl3 (35). ApoA-I and apoB were analyzed with a commercially available assay (Wako Chemicals USA, Inc.) on a Cobas Mira autoanalyzer (Roche Diagnostics).
Postheparin LPL and HL activities were analyzed in plasma collected from fasting subjects at 10 min after intravenous heparin administration (20 U/kg). Enzyme activities were determined by a modification of the method of Boberg and Carlson (36). LPL was determined after inhibition of HL with an antibody raised to purified HL (37). HL was determined after inhibition of LPL with 1 M NaCl. Lipase activities are expressed as micrograms of free fatty acids released per hour per milliliter of postheparin plasma.
Polymorphism detection
A method of template-directed dye terminator incorporation with fluorescence polarization (38) was established to detect the p.185Gly>Cys SNP (rs2075291). PCR primers were GAAGACACCAAGGCCCAGTT (5′) and CCTTCCTCAGTCCCAGTGCC (3′), and the extension primer was GCGTGGTGCACCACACC. The PCRs contained 2.4 ng of dried DNA, 3 μl of PCR primer mix (0.4 μM each primer), and 3 μl of PCR reagent mix with the following protocol: 95°C for 2 min; 35 cycles of 96°C for 20 s, 67°C for 20 s, and 72°C for 30 s; and 7 min at 72°C. The PCR reagent mix was as follows: Platinum Taq, 0.02 μl (5 U/μl; Invitrogen, Carlsbad, CA); 10× buffer, 0.5 μl; MgCl2 (50 mM), 0.35 μl; deoxynucleoside triphosphate (2.5 mM), 0.1 μl; water, 2.03 μl. Following exo-sap cleanup of the PCR product, the extension primer was used for the separate template-directed dye terminator incorporation reaction (38).
Haplotype analysis
Haplotypes were constructed using the program PHASE version 2.1 (39) (http://www.stat.washington.edu/stephens/software.html). The 5′ flanking SNP, rs662799, was detected as described previously (15). The c.−3G>A (rs651821) and p.Ser19Trp (rs3135506) SNPs were determined as restriction fragment-length polymorphisms using BspMI and EaeI, respectively, with PCR primers AGGGGTAACAGGATTTCGGG (5′) and CTACGGAGTTGTCAAGGCGG (3′). The rs34282181 and rs12287066 SNPs were determined by sequencing using PCR primers AGGGGTAACAGGATTTCGGG (5′) and CTACGGAGTTGTCAAGGCGG (3′). The rs2072560 and rs3135507 (p.Val153Met) SNPs were determined by sequencing using PCR primers GCTAGGACAAGAGCCCTCGAC (5′) and CCTTCCTCAGTCCCAGTGCC (3′). The p.Q341H (rs7120555), c.*31C>T (rs619054), and c.*76C>T (rs34089864) SNPs were also determined by sequencing using primers GAGCTCTTCCACCCATACGCCG (5′) and CAGGAGACAGCAGCCCCTTTGG (3′). Sequencing was performed using BigDye Terminator version 3.1 and a 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA).
Throughout this article, we have followed the rules for nomenclature for the description of sequence variations as compiled by the Human Genome Variation Society (40) (http://www.hgvs.org/mutnomen/).
Statistical analysis
Allele and genotype frequencies were determined by the gene-counting method. Hardy-Weinberg equilibrium was assessed for biallelic markers using a Chi-square test. Comparisons between high-TG and low-TG groups were performed using Fisher's exact test for independent qualitative data. Means of log-transformed or normally distributed variables were compared using the independent samples t-test. Odds ratios (ORs), adjusted for other covariables, were determined using logistic regression models. All statistical tests were performed using the program SPSS (SPSS, Inc., Chicago, IL).
In order to evaluate the role of APOA5 genotype, body mass index (BMI), sex, age, diabetes, hypertension, alcohol consumption, and smoking on the binary outcome of hypertriglyceridemia, a logistic model was fit. The following interactions were assessed: APOA5 genotype and BMI, sex, age, diabetes, hypertension, alcohol consumption, and smoking. Backward step-wise regression was conducted manually with predictor variables with P < 0.2 retained for the final fitted model. Model assumptions and fit were assessed by examination of standardized Pearson residuals to identify outlying observations, while model fit was assessed by goodness-of-fit test.
RESULTS
Characteristics of the Chinese-American case and control groups
In this association study of the APOA5 gene c.553G>T (p.185Gly>Cys) SNP, the groups were chosen on the basis of fasting levels of TG in plasma above 150 mg/dl (high TG) or below 150 mg/dl (low TG). The characteristics of the two groups are presented in Table 1. Because there were significant differences in age, sex ratio, BMI, and frequency of diabetes between the groups, we subsequently adjusted the ORs for these parameters. There was an increased incidence of angina and hypertension in the high-TG group, but no differences were seen in the frequency of stroke or family history of CAD. Total cholesterol and VLDL-C were significantly higher in the high-TG group, but there was no difference in LDL-C. As expected, the higher levels of TG in the high-TG group are reflected in higher levels of VLDL-TG. Levels of HDL-C in this group were significantly lower, consistent with the well-established inverse hyperbolic relationship between levels of HDL-C and TG due to the transfer of cholesteryl esters from HDL to TG-rich lipoproteins (41).
TABLE 1.
Characteristics of the Chinese-American groups
| Variable | High TG (n = 152) (mg/dl) | Low TG (n = 148) (mg/dl) | P |
|---|---|---|---|
| Age, years | 54.3 ± 1.5 (152) | 49.4 ± 1.6 (148) | 0.026a |
| Female, % | 47.4 (152) | 62.8 (148) | 0.007b |
| BMI, kg/m2 | 24.7 ± 0.4 (139) | 23.1 ± 0.4 (136) | <0.001a |
| Total cholesterol | 276.8 ± 9.0 (152) | 232.2 ± 5.6 (148) | <0.001a |
| TG | 477.2 ± 65.3 (152) | 101.7 ± 2.3 (148) | –c |
| VLDL-C | 96.4 ± 16.0 (85) | 14.2 ± 1.3 (75) | <0.001a |
| VLDL-TG | 466.2 ± 102.1 (85) | 52.5 ± 2.8 (75) | <0.001a |
| LDL-C | 151.2 ± 5.3 (133) | 154.6 ± 5.5 (146) | 0.662a |
| LDL-TG | 55.4 ± 3.4 (85) | 29.7 ± 1.1 (75) | <0.001a |
| HDL-C | 45.4 ± 1.2 (144) | 60.7 ± 1.6 (146) | <0.001a |
| Myocardial infarction, % | 8.7 (150) | 3.4 (146) | 0.059b |
| Angina, % | 12.2 (147) | 4.9 (142) | 0.027b |
| Family history of coronary heart disease, % | 28.5 (130) | 30.3 (132) | 0.744b |
| Stroke, % | 4.0 (149) | 4.7 (148) | 0.767b |
| Diabetes, % | 18.2 (148) | 6.8 (147) | 0.003b |
| Hypertension, % | 41.6 (149) | 22.4 (147) | <0.001b |
BMI, body mass index; -C, -cholesterol; TG, triglyceride. Values shown are ± SEM, with the numbers of subjects in each instance in parentheses.
Calculated by unpaired Student's t-test (TG values were log-transformed prior to testing).
Calculated by Chi-square test.
Use of this variable as a case selection criterion precludes the reporting of a statistical significance.
Rare allele frequencies
Table 2 reveals the striking, and highly significant, 4-fold increased frequency of the c.553T allele in the Chinese-American high-TG group compared with the low-TG group (15.1% vs. 3.7%). There was an even larger, 5.5-fold, higher frequency in males. The 2.6-fold increase in the T allele frequency with females was less significant. Of note, the frequency of the T allele was found to be 4.5% in a population of 101 unselected free-living Chinese Americans recruited at a San Francisco Chinatown health fair (data not shown). The unadjusted OR was 4.45 (95% confidence interval, 2.18–9.07; P < 0.001) (see supplementary Table I). When adjusted for age, sex, and BMI, the OR was 4.40 (95% confidence interval, 1.99–9.74; P < 0.001) (data not shown). When diabetes was added as an additional covariable, the OR was 4.27 (95% confidence interval, 1.91–9.56; P < 0.001).
TABLE 2.
APOA5 c.553G>T allele and genotype frequencies in Chinese-American subjects
| Subjects | High TG | n | Low TG | n | Pa |
|---|---|---|---|---|---|
| Female and male | 152 | 148 | |||
| GG | 0.737 | 112 | 0.926 | 137 | |
| GT | 0.224 | 34 | 0.074 | 11 | <0.001 |
| TT | 0.039 | 6 | 0 | 0 | |
| GT + TT | 0.263 | 40 | 0.074 | 11 | <0.001 |
| T allele | 0.151 | 46 | 0.037 | 11 | <0.001 |
| Female | 72 | 93 | |||
| GG | 0.819 | 59 | 0.925 | 86 | |
| GT | 0.167 | 12 | 0.075 | 7 | 0.093 |
| TT | 0.014 | 1 | 0 | 0 | |
| GT + TT | 0.181 | 13 | 0.075 | 7 | 0.054 |
| T allele | 0.097 | 14 | 0.038 | 7 | 0.039 |
| Male | 80 | 55 | |||
| GG | 0.663 | 53 | 0.927 | 51 | |
| GT | 0.275 | 22 | 0.073 | 4 | 0.001 |
| TT | 0.063 | 5 | 0 | 0 | |
| GT + TT | 0.338 | 27 | 0.073 | 4 | <0.001 |
| T allele | 0.2 | 32 | 0.036 | 4 | <0.001 |
P values were calculated by Fisher's exact test.
Non-Chinese Asian-American subjects
The characteristics of the replication cohort of non-Chinese American-Asian groups are given in supplementary Table II. Our results with Chinese-American subjects were confirmed in this cohort. The T allele frequency was 2.5-fold higher in the high-TG group (13.7% vs. 5.4%; Chi-square = 20.2; P < 0.0001). The OR was 2.75 (95% confidence interval, 1.32–5.74; P = 0.007) (see supplementary Table I).
The genotypes of all groups (females, males, high TG, and low TG) were found to be in Hardy-Weinberg equilibrium.
Genetic association of lipids and lipoproteins
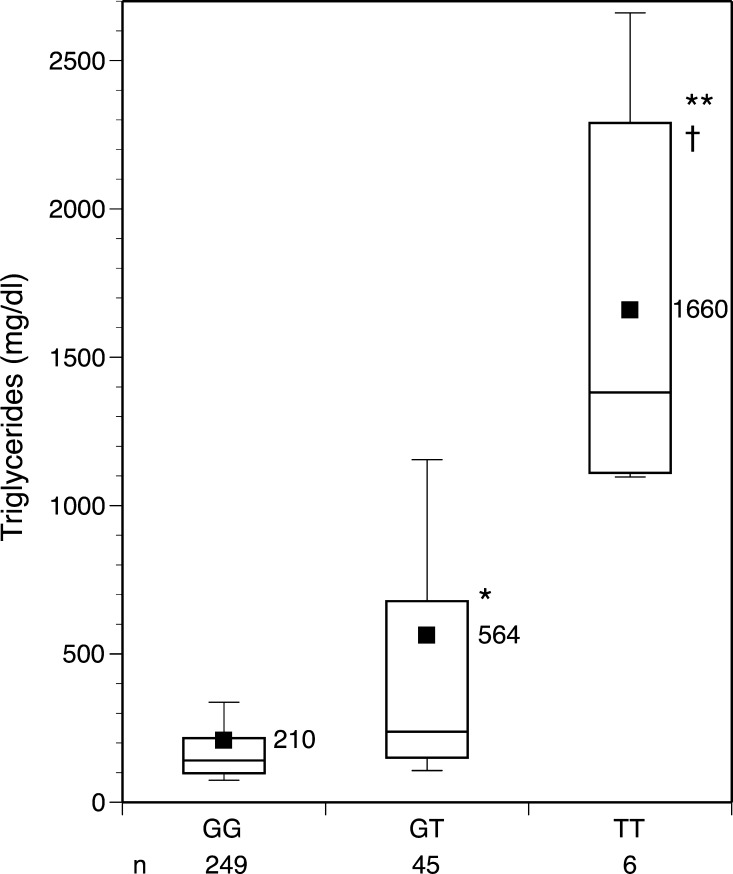
The association of the c.553T genotype with levels of TG in the complete Chinese-American group is presented in Fig. 1, which shows a pronounced gene-dosage effect. In the high-TG and low-TG groups, separately, we evaluated the association of the SNP on plasma lipid and lipoprotein concentrations. In the high-TG group, the plasma levels of total cholesterol and TG, as well as VLDL-C and VLDL-TG, were notably and significantly elevated in those subjects carrying the T allele, again with a clear gene-dosage effect (Table 3). The ratio of cholesterol to TG in VLDL, however, was unchanged. The presence of the T allele was associated with a decrease in LDL-C and an increase in LDL-TG. The decrease in HDL-C in carriers, again with a gene-dosage effect, reflects the transfer of cholesteryl esters from HDL to TG-rich lipoproteins, as mentioned above. In the low-TG group, the T allele was associated with doubled levels of VLDL-C and a 14% increase in TG (Table 4), but this was not significant, possibly reflecting low power.
Fig. 1.
Association of the APOA5 c.553G>T single nucleotide polymorphism (SNP; p.185Gly>Cys) with plasma levels of triglycerides in the Chinese-American population. The mean values are shown next to the boxes, which show the 25th, 50th, and 75th percentiles (the error bars show outlier caps). * GG versus GT (P < 0.0001); ** GG versus TT (P < 0.0001); † GT versus TT (P < 0.001) calculated from log-transformed data. The T allele frequency is 9.5%.
TABLE 3.
Chinese-American high-TG group plasma lipids, age, and BMI, by APOA5 c.553G>T genotype
| Variable | GG | GT | TT | P |
|---|---|---|---|---|
| Age, years | 51.0 ± 1.8 (112) | 52.2 ± 2.5 (34) | 49.0 ± 5.8 (6) | 0.895 |
| BMI, kg/m2 | 24.9 ± 0.4 (105) | 24.4 ± 0.5 (29) | 23.8 ± 1.0 (6) | 0.715 |
| Total cholesterol | 270 ± 9 (112) | 273 ± 23 (34) | 422 ± 30 (6) | 0.001a |
| Total TGs | 343 ± 66 (112) | 709 ± 160 (34) | 1660 ± 274 (6) | <0.001a |
| VLDL-C | 66 ± 16 (61) | 130 ± 41 (19) | 333 ± 25 (5) | <0.001a |
| VLDL-TG | 326 ± 106 (61) | 632 ± 262 (19) | 1546 ± 331 (5) | <0.001a |
| LDL-C | 163 ± 6 (105) | 113 ± 11 (23) | 80 ± 11 (5) | <0.001a |
| LDL-TG | 47.9 ± 2.5 (61) | 61.2 ± 8.3 (19) | 125 ± 21 (5) | <0.001a |
| HDL-C | 47.8 ± 1.4 (108) | 39.1 ± 2.2 (31) | 32.2 ± 7.1 (5) | 0.001 |
All lipid values are mg/dl and are expressed as means ± SEM. P values were calculated by ANOVA. Numbers of subjects are in parentheses.
Calculated from log-transformed data.
TABLE 4.
Chinese-American low-TG group plasma lipids, age, and BMI, by APOA5 c.553G>T genotype
| Variable | GG | GT | P |
|---|---|---|---|
| Age, years | 47.0 ± 1.7 (137) | 55.9 ± 6.5 (11) | 0.149 |
| BMI, kg/m2 | 23.1 ± 0.4 (127) | 22.2 ± 0.8 (9) | 0.545 |
| Total cholesterol | 232 ± 6 (137) | 234 ± 15 (11) | 0.939 |
| Total TGs | 101 ± 2.4 (137) | 115 ± 7.2 (11) | 0.101 |
| VLDL-TC | 13.6 ± 1.1 (72) | 29.0 ± 15.5 (3) | 0.064a |
| VLDL-TG | 52.0 ± 2.8 (72) | 63.0 ± 18.0 (3) | 0.444 |
| LDL-C | 155 ± 6 (136) | 145 ± 15 (10) | 0.636 |
| LDL-TG | 29.6 ± 1.1 (72) | 30.7 ± 6.5 (3) | 0.856 |
| HDL-C | 60.4 ± 1.6 (136) | 65.1 ± 4.5 (10) | 0.446 |
All lipid values are mg/dl and are expressed as means ± SEM. Numbers of subjects are in parentheses.
Calculated from log-transformed data.
The effects of the polymorphism on plasma TG and HDL-C in the four non-Chinese Asian population subgroups in this study are shown in Table 5. The frequency of the T allele was highest in the Japanese group and lowest in the Southeast Asians. In all four groups, the levels of TG were higher in the heterozygotes than in the more common GG homozygotes, and these differences were significant in the Pacific Islander and Southeast Asian subjects. Levels of HDL-C were lower for heterozygotes in all four populations, and this was significant in the Pacific Islander and Korean groups. It should be noted that the numbers of people in each of these subgroups was considerably lower than for the Chinese-American population. The overall difference in TG levels between the GG and GT groups is similar to that seen in the Chinese-American population (Fig. 1). One Pacific Islander, one Japanese, and one Korean individual were homozygous for the T allele. All three were severely hypertriglyceridemic, as noted below. The polymorphism was not seen in South Asians (India or Pakistan) and was only present at an extremely low frequency in Caucasians: among 779 non-Asian individuals, only three carriers were found, two Caucasian and one Hispanic.
TABLE 5.
Effect of APOA5 c.553G>T heterozygosity on lipid profiles in Pacific Islander, Southeast Asian, Japanese-American, and Korean-American populations
| Variable | T Allele Frequency (%) | GG | GT | P |
|---|---|---|---|---|
| Pacific Islander | 7.5 | |||
| Total TGs | 221 ± 24 (83) | 614 ± 137 (14) | <0.001a | |
| HDL-C | 51.1 ± 1.9 | 40.9 ± 4.2 (14) | 0.044 | |
| Southeast Asian | 5.1 | |||
| Total TGs | 217 ± 26 (53) | 657 ± 304 (6) | 0.007a | |
| HDL-C | 49.9 ± 2.8 (51) | 47.8 ± 6.2 (4) | 0.837 | |
| Japanese | 13.5 | |||
| Total TGs | 238 ± 50 (42) | 380 ± 159 (19) | 0.423a | |
| HDL-C | 63.8 ± 4.6 (41) | 52.8 ± 5.1 (18) | 0.161 | |
| Korean | 9.6 | |||
| Total TGs | 413 ± 160 (16) | 1043 ± 566 (4) | 0.056a | |
| HDL-C | 51.3 ± 3.8 (16) | 32.2 ± 6.8 (4) | 0.036 |
All lipid values are mg/dl and are expressed as means ± SEM. Numbers of subjects are in parentheses.
Calculated from log-transformed data.
The characteristics of all 11 subjects identified as homozygotes are given in Table 6; all had pronounced hypertriglyceridemia. Subject 2 developed hypertension at age 62 and had a myocardial infarction at age 77. Subjects 6 and 8 developed hypertension at ages 32 and 58, respectively. In addition, subject 8 developed diabetes at age 45. Little clinical information was available for subjects 5, 7, and 9. One homozygote was Caucasian. This was somewhat surprising, given the overall very low frequency of the T allele in this ethnic group.
TABLE 6.
Characteristics of APOA5 c.553G>T homozygous patients
| Subject | Sex | Ethnic Origin | BMI | Age | Total Cholesterol | TG | VLDL-C | VLDL-TG | LDL-C | LDL-TG | HDL-C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Chinese | 25.8 | 48 | 405 | 1,110 | 288 | 990 | 81 | 85 | 36 |
| 2 | Male | Chinese | 22.9 | 75 | 306 | 1,370 | |||||
| 3 | Male | Chinese | 21.5 | 49 | 519 | 1,393 | 371 | 1,225 | 120 | 138 | 28 |
| 4 | Male | Chinese | 27.4 | 51 | 485 | 2,702 | 405 | 2,483 | 61 | 172 | 19 |
| 5 | Male | Chinese | 24.1 | 33 | 405 | 2,290 | 328 | 2,190 | 57 | 68 | 20 |
| 6 | Female | Chinese | 21.2 | 39 | 414 | 1,095 | 273 | 845 | 83 | 164 | 58 |
| 7 | Male | Chinese | 25.0 | 49 | 133 | 687 | |||||
| 8 | Male | Japanese | 29.2 | 34 | 610 | 4,968 | |||||
| 9 | Male | Korean | — | 25 | 676 | 2,220 | 538 | 1,980 | 52 | 125 | 25 |
| 10 | Female | Pacific Islander | 19.9 | 51 | 543 | 5,040 | |||||
| 11 | Female | White European | — | 31 | 649 | 2,339 | 543 | 1,953 | 47 | 83 | 33 |
| Mean | 24.1 | 44.1 | 468 | 2,292 | 392 | 1,667 | 71.6 | 119 | 31.3 | ||
| SEM | 1.0 | 4.2 | 48 | 447 | 42 | 242 | 9.6 | 16 | 5.0 | ||
| n | 9 | 11 | 11 | 11 | 7 | 7 | 7 | 7 | 7 |
Lipid measurements are expressed as mg/dl, and BMI is expressed as kg/m2.
We analyzed the combined data from the two studies in an attempt to explain the large range of TG values observed in heterozygous individuals (see supplementary Table IV). In a regression model, we included, in addition to APOA5 genotype, several potential confounding variables: BMI, sex, age, diabetes, hypertension, alcohol consumption, and smoking. The analysis revealed that only sex (i.e., male sex) was a modest positive confounder. Although BMI, diabetes, and hypertension were all independent predictors of TG, they did not affect the relationship of APOA5 genotype with TG. Neither alcohol consumption nor smoking was a predictor of TG, and neither affected the association between APOA5 genotype and TG. Although the estimate of the coefficient for age was not significant, this variable was retained in the model to maintain validity.
Haplotype analysis
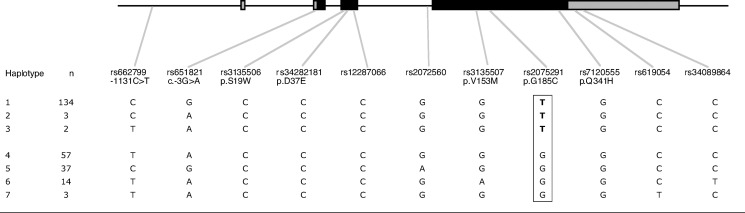
Haplotypes were inferred for all of the 125 c.553T carriers that we identified; in 90% of cases, this was with >92% certainty, and in 10% of cases, it was with 71–91% confidence. One haplotype (haplotype 1) accounted for almost all T alleles (Fig. 2). All Chinese-American carriers displayed this haplotype except one, who had haplotype 2. Of the other two subjects with haplotype 2, one was a Pacific Islander and the other was of mixed ancestry: Pacific Islander and Japanese. The two subjects with haplotype 3 were both Pacific Islanders. All 11 T homozygotes were homozygous for haplotype 1.
Fig. 2.
Schematic diagram of the APOA5 gene showing the positions of 11 SNPs genotyped. Haplotypes were constructed using the program PHASE version 2.1. Three haplotypes, 1, 2, and 3, carried the c.553T variant.
One-third of c.553T heterozygotes had, as the other allele, haplotype 5 (Fig. 2), which has the less common C in SNP rs662799 (−1131C>T). Further analysis of heterozygous carriers revealed that those with haplotype 5 had the highest TGs compared with those with haplotypes 4, 6, or 7 (see supplementary Table III). Those with haplotype 5 had a mean TG of 832 ± 216 mg/dl, compared with 444 ± 70 mg/dl for subjects with the other haplotypes (P = 0.018). When haplotype 5 was the second allele, levels of VLDL-C and VLDL-TG were also significantly elevated and HDL-C was significantly lower (see supplementary Table III). Figure 2 shows that the −1131 and c.−3G>A SNPs were in very strong, but not complete, linkage disequilibrium, as reported previously in a group of Japanese Americans (22).
Additional biochemical studies
Further, in-depth biochemical studies were performed on plasma samples from a subset of c.553T carriers. For comparison, a random group of GG homozygotes was selected from the total Asian population set. Individuals for this study were included irrespective of their levels of TG. These results are presented in Table 7. As before, we found striking, and highly significant, increases in levels of TG. As before, HDL-C was decreased, here by 23%, but the amount of apoA-I was decreased by only 10%, and not significantly. The levels of apoC-II, apoC-III, and apoE were moderately and significantly increased (by 61, 42, and 52%, respectively) in the carrier group, reflecting the higher levels of TG-rich lipoproteins. In contrast, apoA-V was increased by only 13%.
TABLE 7.
Multiple biochemical parameters and characteristics of a subgroup of subjects
| Variable | GG (n = 28) | GT/TT (n = 42) | P |
|---|---|---|---|
| Age, years | 56.0 ± 3.5 (28) | 53.2 ± 2.9 (42) | 0.536 |
| Female, % | 60.7 (28) | 40.5 (42) | 0.097 |
| Total cholesterol | 230.8 ± 8.3 (28) | 249.6 ± 11.4 (42) | 0.233 |
| TGsa | 214.0 ± 46.9 (28) | 452.3 ± 78.6 (42) | <0.001 |
| LDL-C | 143.5 ± 7.2 (23) | 122.7 ± 7.6 (31) | 0.060 |
| HDL-C | 58.1 ± 4.4 (25) | 44.6 ± 2.6 (36) | 0.007 |
| ApoA-I | 141.7 ± 5.6 (24) | 127.9 ± 4.6 (36) | 0.064 |
| ApoA-V,a ng/ml | 206.2 ± 28.5 (24) | 232.9 ± 40.2 (36) | 0.972 |
| ApoB | 93.8 ± 3.5 (24) | 90.2 ± 2.8 (36) | 0.427 |
| ApoC-IIa | 4.6 ± 0.5 (28) | 7.4 ± 0.6 (42) | <0.001 |
| ApoC-IIIa | 10.2 ± 0.7 (28) | 14.5 ± 0.9 (42) | <0.001 |
| ApoEa | 4.4 ± 0.2 (28) | 6.7 ± 0.5 (42) | <0.001 |
| Angiopoietin-like protein 3,a ng/ml | 158.6 ± 14.9 (28) | 202.2 ± 31.6 (42) | 0.287 |
ApoA-I, apolipoprotein A-I. Values are ±SEM and are mg/dl unless indicated otherwise; numbers of observations are in parentheses. P values were calculated by unpaired Student's t-test, except for the female value, which was by Chi-square test.
These values were log-transformed prior to testing.
Postheparin LPL activity measured in 7 c.553T carriers was 34.5% lower than in 31 low-TG controls (3.8 ± 1.1 and 5.8 ± 0.3 U/ml, respectively; P = 0.027). In contrast, there was no difference in hepatic lipase activity between carriers and controls (4.8 ± 0.3 and 4.8 ± 0.8 U/ml, respectively; P = 0.987).
DISCUSSION
We successfully replicated the previously reported association of an apoA-V polymorphism (c.553G>T; p.185Gly>Cys) with plasma levels of TG in China and Taiwan (25, 26). Our population was of Chinese descent living in the United States. The presence of the less common T (cysteine) allele conferred a substantial 4.45 times greater risk of hypertriglyceridemia. We found that this association persisted in a non-Chinese cohort consisting of four other Asian-American ethnic groups: those of Japanese, Korean, Southeast Asian, and Pacific Islander ancestry. Overall, in both populations, there was a marked association with the presence of the T allele with a pronounced gene-dosage effect. These differences were more pronounced in men than in women. One in sixty (1.7%) of the Asian subjects we studied were homozygous carriers of the c.553T allele; all had severe hypertriglyceridemia. The 4.5% T allele frequency that we found in 101 unselected Chinese Americans is somewhat higher than the 2.2% reported in the HapMap project for 45 Han Chinese in Beijing (http://www.hapmap.org). Based on our data, homozygosity for the T allele would be expected at 1 in 500 free-living Chinese Americans.
What needs to be explained, given the markedly increased frequency of the c.553T allele and the prominent effect on levels of TG in the high-TG group, is the lack of effect on lipids and lipoprotein parameters in the low-TG groups. In the Chinese-American low-TG group, the effect was not significant, although it was similar in magnitude to the report of Kao et al. (25) in a Taiwanese control population, in which heterozygotes had 15% higher TG. This incomplete dominance could be due to an interaction between the polymorphism and other genetic factors, which could be determined in family studies. Environmental effects and diet may also play a role. Our analysis showed that while BMI, diabetes, and hypertension were all predictors of TG, they did not have an impact on the correlation of APOA5 genotype with TG. It is worth noting that the impact of the APOA5 genotype is two to three times greater than any of the other predictors. In Caucasian or predominantly Caucasian populations (6, 15–23), and in Chinese populations (42, 43), it has been shown that the −1131C variant is associated with a significant increase in levels of TG, although the effects were considerably lower in magnitude than seen here with the c.553G>T SNP. Our present study shows that, in combination with c.553T, the −1131C variant has a significant compounding effect.
The Chinese-American high-TG group showed a marked decrease in HDL-C associated with the presence of the T allele. We estimated, based on the curvilinear relationship between HDL-C and TG as described by Meyers, Phillips, and Havel (41), that one would expect a 16% lower HDL-C in the GT group compared with the GG group, solely due to the effect of mass transfer associated with the difference in TG (709 vs. 343 mg/dl) (Table 3). We actually observed 18% lower HDL-C; hence, the major part of the associated lower HDL-C can be explained by this effect alone.
Some previous evidence points to apoA-V being an activator of LPL (44, 45). We observed significantly lower postheparin LPL activity in c.553T carriers. Thus, given the essentially unchanged level of apoA-V in the carrier group, it is likely that the cysteine-185 apoA-V variant may be less efficient at activating the enzyme. This activation, it should be pointed out, is not direct but by some as yet unclear indirect mechanism (46). We postulated that apoA-V could affect LPL activity indirectly by affecting the level of angptl3. Angptl3 has been implicated as a regulator of TG metabolism (47), and recently, genetic variants near to the ANGPTL3 gene were associated with levels of plasma TG (48, 49). Angptl3 decreases VLDL-TG clearance via the inhibition of lipolysis by LPL (50). Our results (Table 7) show that levels of angptl3 were indeed higher in c.553T carriers, but not significantly so.
There was a marginal, but not significant, decrease in the level of apoA-I and modest, significant increases in apoE, apoC-II, and apoC-III that probably reflect an increase in the surface area of TG-rich lipoproteins.
Our data show clearly that the presence of the APOA5 p.185Gly>Cys variant results in considerable effects on TG levels in the Asian populations that we have studied. Among the 65 subjects with TG above 500 mg/dl, 60% were carriers: 45% heterozygous and 15% homozygous. Of the 29 with TG above 1,000 mg/dl, 79% were carriers: 45% heterozygous and 34% homozygous. All but one of the homozygotes we identified had levels of TG exceeding 1,000 mg/dl. Clearly, at the frequency we have observed, tens of millions of people worldwide are carriers with increased risk of developing hypertriglyceridemia. It was suggested recently that individuals with TG levels above 1,000–1,500 mg/dl should be treated with fibrates to lower the risk of pancreatitis (51). In the case of T allele carriers with such TG levels, we believe that it would be appropriate to screen close relatives to alert the carriers of their increased risk and to offer them intervention if necessary. Future family- and population-based studies are needed to determine, more precisely, the discriminative ability of the polymorphism to identify family members with an increased risk of severe hypertriglyceridemia.
Supplementary Material
Published, JLR Papers in Press, April 25, 2008.
Footnotes
This work was supported by grants from the American Heart Association (Grants 0655195Y to C.R.P. and 0465005Y to B.E.A.), National Institutes of Health National Center for Research Resources Grant KL2 RR-024130 to B.E.A., a Hellman Family Award (to C.R.P. and B.E.A), a UCSF Academic Senate Award (to C.R.P.), the Leducq Foundation, the Joseph Drown Foundation (to M.J.M.), and by gifts from Donald Yellon and the Mildred V. Strouss Charitable Trust.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four tables.
References
- 1.Brewer H. B. 1999. Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am. J. Cardiol. 83 3F–12F. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg H. N. 1999. Identification and treatment of hypertriglyceridemia as a risk factor for coronary heart disease. Curr. Cardiol. Rep. 1 233–237. [DOI] [PubMed] [Google Scholar]
- 3.Austin M. A. 1999. Epidemiology of hypertriglyceridemia and cardiovascular disease. Am. J. Cardiol. 83 13F–16F. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard B. G., M. Benn, P. Schnohr, and A. Tybjaerg-Hansen. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. J. Am. Med. Assoc. 298 299–308. [DOI] [PubMed] [Google Scholar]
- 5.van der Vliet H. N., M. G. Sammels, A. C. Leegwater, J. H. Levels, P. H. Reitsma, W. Boers, and R. A. Chamuleau. 2001. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 276 44512–44520. [DOI] [PubMed] [Google Scholar]
- 6.Pennacchio L. A., M. Olivier, J. A. Hubacek, J. C. Cohen, D. R. Cox, J. C. Fruchart, R. M. Krauss, and E. M. Rubin. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294 169–173. [DOI] [PubMed] [Google Scholar]
- 7.Alborn W. E., M. G. Johnson, M. J. Prince, and R. J. Konrad. 2006. Definitive N-terminal protein sequence and further characterization of the novel apolipoprotein A5 in human serum. Clin. Chem. 52 514–517. [DOI] [PubMed] [Google Scholar]
- 8.Allayee H., B. E. Aouizerat, R. M. Cantor, G. M. Dallinga-Thie, R. M. Krauss, C. D. Lanning, J. I. Rotter, A. J. Lusis, and T. W. de Bruin. 1998. Families with familial combined hyperlipidemia and families enriched for coronary artery disease share genetic determinants for the atherogenic lipoprotein phenotype. Am. J. Hum. Genet. 63 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. C., Z. Wang, S. M. Grundy, M. R. Stoesz, and R. Guerra. 1994. Variation at the hepatic lipase and apolipoprotein AI/CIII/AIV loci is a major cause of genetically determined variation in plasma HDL cholesterol levels. J. Clin. Invest. 94 2377–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallinga-Thie G. M., M. van Linde-Sibenius Trip, J. I. Rotter, R. M. Cantor, X. Bu, A. J. Lusis, and T. W. de Bruin. 1997. Complex genetic contribution of the Apo AI-CIII-AIV gene cluster to familial combined hyperlipidemia. Identification of different susceptibility haplotypes. J. Clin. Invest. 99 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessling A. M., K. Berg, E. Mockleby, and S. E. Humphries. 1986. DNA polymorphisms around the apo AI gene in normal and hyperlipidaemic individuals selected for a twin study. Clin. Genet. 29 485–490. [DOI] [PubMed] [Google Scholar]
- 12.Kessling A. M., M. N. Nanjee, N. E. Miller, and S. E. Humphries. 1988. Variations in the apolipoprotein AI-CIII-AIV gene region and in lecithin:cholesterol acyltransferase concentration are determinants of plasma cholesterol concentrations. Atherosclerosis. 70 13–19. [DOI] [PubMed] [Google Scholar]
- 13.Wojciechowski A. P., M. Farrall, P. Cullen, T. M. E. Wilson, J. D. Bayliss, B. Farren, B. A. Griffin, M. J. Caslake, C. J. Packard, J. Shepherd, et al. 1991. Familial combined hyperlipidaemia linked to the apolipoprotein AI-CIII-AIV gene cluster on chromosome 11q23-q24. Nature. 349 161–164. [DOI] [PubMed] [Google Scholar]
- 14.Xu C. F., P. Talmud, H. Schuster, R. Houlston, G. Miller, and S. Humphries. 1994. Association between genetic variation at the APO AI-CIII-AIV gene cluster and familial combined hyperlipidaemia. Clin. Genet. 46 385–397. [DOI] [PubMed] [Google Scholar]
- 15.Aouizerat B. E., M. Kulkarni, D. Heilbron, D. Drown, S. Raskin, C. R. Pullinger, M. J. Malloy, and J. P. Kane. 2003. Genetic analysis of a polymorphism in the human apoA-V gene: effect on plasma lipids. J. Lipid Res. 44 1167–1173. [DOI] [PubMed] [Google Scholar]
- 16.Talmud P. J., E. Hawe, S. Martin, M. Olivier, G. J. Miller, E. M. Rubin, L. A. Pennacchio, and S. E. Humphries. 2002. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum. Mol. Genet. 11 3039–3046. [DOI] [PubMed] [Google Scholar]
- 17.Endo K., H. Yanagi, J. Araki, C. Hirano, K. Yamakawa-Kobayashi, and S. Tomura. 2002. Association found between the promoter region polymorphism in the apolipoprotein A-V gene and the serum triglyceride level in Japanese schoolchildren. Hum. Genet. 111 570–572. [DOI] [PubMed] [Google Scholar]
- 18.Evans D., A. Buchwald, and F. U. Beil. 2003. The single nucleotide polymorphism −1131T>C in the apolipoprotein A5 (APOA5) gene is associated with elevated triglycerides in patients with hyperlipidemia. J. Mol. Med. 81 645–654. [DOI] [PubMed] [Google Scholar]
- 19.Mar R., P. Pajukanta, H. Allayee, M. Groenendijk, G. Dallinga-Thie, R. M. Krauss, J. S. Sinsheimer, R. M. Cantor, T. W. de Bruin, and A. J. Lusis. 2004. Association of the APOLIPOPROTEIN A1/C3/A4/A5 gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circ. Res. 94 993–999. [DOI] [PubMed] [Google Scholar]
- 20.Martin S., V. Nicaud, S. E. Humphries, and P. J. Talmud. 2003. Contribution of APOA5 gene variants to plasma triglyceride determination and to the response to both fat and glucose tolerance challenges. Biochim. Biophys. Acta. 1637 217–225. [DOI] [PubMed] [Google Scholar]
- 21.Wright W. T., I. S. Young, D. P. Nicholls, C. Patterson, K. Lyttle, and C. A. Graham. 2006. SNPs at the APOA5 gene account for the strong association with hypertriglyceridaemia at the APOA5/A4/C3/A1 locus on chromosome 11q23 in the Northern Irish population. Atherosclerosis. 185 353–360. [DOI] [PubMed] [Google Scholar]
- 22.Austin M. A., P. J. Talmud, F. M. Farin, D. A. Nickerson, K. L. Edwards, D. Leonetti, M. J. McNeely, H. M. Viernes, S. E. Humphries, and W. Y. Fujimoto. 2004. Association of apolipoprotein A5 variants with LDL particle size and triglyceride in Japanese Americans. Biochim. Biophys. Acta. 1688 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Dallinga-Thie G. M., A. van Tol, H. Hattori, L. C. van Vark-van der Zee, H. Jansen, and E. J. Sijbrands. 2006. Plasma apolipoprotein A5 and triglycerides in type 2 diabetes. Diabetologia. 49 1505–1511. [DOI] [PubMed] [Google Scholar]
- 24.Saxena R., B. F. Voight, V. Lyssenko, N. P. Burtt, P. I. de Bakker, H. Chen, J. J. Roix, S. Kathiresan, J. N. Hirschhorn, M. J. Daly, et al. 2007. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 316 1331–1336. [DOI] [PubMed] [Google Scholar]
- 25.Kao J. T., H. C. Wen, K. L. Chien, H. C. Hsu, and S. W. Lin. 2003. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum. Mol. Genet. 12 2533–2539. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y., P. Sun, D. Guo, A. Ferro, Y. Ji, Q. Chen, and L. Fan. 2006. A genetic variant c.553G > T in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and altered triglyceride levels in a Chinese population. Atherosclerosis. 185 433–437. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman D., S. G. Ellis, C. M. Rowland, M. J. Malloy, M. M. Luke, O. A. Iakoubova, C. R. Pullinger, J. Cassano, B. E. Aouizerat, R. G. Fenwick, et al. 2005. Identification of four gene variants associated with myocardial infarction. Am. J. Hum. Genet. 77 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). J. Am. Med. Assoc. 285: 2486–2497. [DOI] [PubMed]
- 29.Kane J. P., M. J. Malloy, T. A. Ports, N. R. Phillips, J. C. Diehl, and R. J. Havel. 1990. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimens. J. Am. Med. Assoc. 264 3007–3012. [PubMed] [Google Scholar]
- 30.Havel R. J., H. A. Eder, and J. H. Bragdon. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnick G. R., J. Benderson, and J. J. Albers. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 28 1379–1388. [PubMed] [Google Scholar]
- 32.Friedewald W. T., R. I. Levy, and D. S. Fredrickson. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18 499–502. [PubMed] [Google Scholar]
- 33.Ishihara M., T. Kujiraoka, T. Iwasaki, M. Nagano, M. Takano, J. Ishii, M. Tsuji, H. Ide, I. P. Miller, N. E. Miller, et al. 2005. A sandwich enzyme-linked immunosorbent assay for human plasma apolipoprotein A-V concentration. J. Lipid Res. 46 2015–2022. [DOI] [PubMed] [Google Scholar]
- 34.Takeichi S., Y. Nakajima, N. Yukawa, T. Saito, Y. Seto, X. L. Huang, T. Kusakabe, Z. B. Jin, I. Hasegawa, T. Nakano, et al. 2001. Plasma triglyceride-rich lipoprotein remnants as a risk factor of ‘Pokkuri disease.’ Leg. Med. (Tokyo). 3 84–94. [DOI] [PubMed] [Google Scholar]
- 35.Miida, T., U. Seino, O. Miyazaki, O. Hanyu, S. Hirayama, T. Saito, Y. Ishikawa, S. Akamatsu, T. Nakano, K. Nakajima, et al. 2008. Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling. Atherosclerosis. Epub ahead of print. February 13, 2008; doi:10.1016/j.atherosclerosis.2007.12.031. [DOI] [PubMed]
- 36.Boberg J., and L. A. Carlson. 1964. Determination of heparin-induced lipoprotein lipase activity in human plasma. Clin. Chim. Acta. 10 420–427. [DOI] [PubMed] [Google Scholar]
- 37.Frost P. H., V. G. Shore, and R. J. Havel. 1982. Purification of canine post-heparin hepatic lipase. Biochim. Biophys. Acta. 712 71–78. [DOI] [PubMed] [Google Scholar]
- 38.Hsu, T. M., X. Chen, S. Duan, R. D. Miller, and P. Y. Kwok. 2001. Universal SNP genotyping assay with fluorescence polarization detection. Biotechniques. 31: 560, 562, 564–568. [DOI] [PubMed]
- 39.Stephens M., N. J. Smith, and P. Donnelly. 2001. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Dunnen J. T., and S. E. Antonarakis. 2000. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum. Mutat. 15 7–12. [DOI] [PubMed] [Google Scholar]
- 41.Meyers L., N. Phillips, and R. Havel. 1976. Mathematical evaluation of methods for estimation of the concentration of the major lipid components of human serum lipoproteins. J. Lab. Clin. Med. 88 491–505. [PubMed] [Google Scholar]
- 42.Bi N., S. K. Yan, G. P. Li, Z. N. Yin, and B. S. Chen. 2004. A single nucleotide polymorphism −1131T>C in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and alters triglyceride metabolism in Chinese. Mol. Genet. Metab. 83 280–286. [DOI] [PubMed] [Google Scholar]
- 43.Baum L., B. Tomlinson, and G. N. Thomas. 2003. APOA5–1131T>C polymorphism is associated with triglyceride levels in Chinese men. Clin. Genet. 63 377–379. [DOI] [PubMed] [Google Scholar]
- 44.van Dijk K. W., P. C. Rensen, P. J. Voshol, and L. M. Havekes. 2004. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr. Opin. Lipidol. 15 239–246. [DOI] [PubMed] [Google Scholar]
- 45.Merkel M., B. Loeffler, M. Kluger, N. Fabig, G. Geppert, L. A. Pennacchio, A. Laatsch, and J. Heeren. 2005. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J. Biol. Chem. 280 21553–21560. [DOI] [PubMed] [Google Scholar]
- 46.Lookene A., J. A. Beckstead, S. Nilsson, G. Olivecrona, and R. O. Ryan. 2005. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J. Biol. Chem. 280 25383–25387. [DOI] [PubMed] [Google Scholar]
- 47.Koishi R., Y. Ando, M. Ono, M. Shimamura, H. Yasumo, T. Fujiwara, H. Horikoshi, and H. Furukawa. 2002. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30 151–157. [DOI] [PubMed] [Google Scholar]
- 48.Willer C. J., S. Sanna, A. U. Jackson, A. Scuteri, L. L. Bonnycastle, R. Clarke, S. C. Heath, N. J. Timpson, S. S. Najjar, H. M. Stringham, et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kathiresan S., O. Melander, C. Guiducci, A. Surti, N. P. Burtt, M. J. Rieder, G. M. Cooper, C. Roos, B. F. Voight, A. S. Havulinna, et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizugawa T., M. Ono, M. Shimamura, K. Yoshida, Y. Ando, R. Koishi, K. Ueda, T. Inaba, H. Minekura, T. Kohama, et al. 2002. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 277 33742–33748. [DOI] [PubMed] [Google Scholar]
- 51.Brunzell J. D. 2007. Clinical practice. Hypertriglyceridemia. N. Engl. J. Med. 357 1009–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.