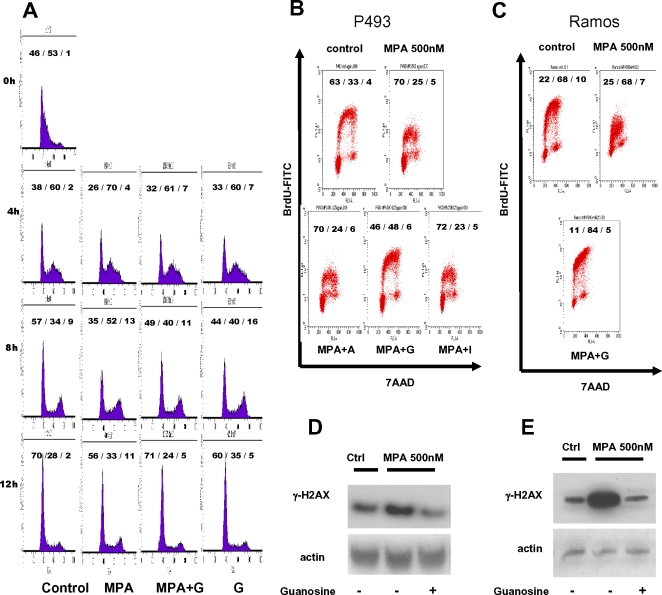
Figure 8. Mycophenolic acid (MPA) slows DNA replication in P493-6 cells.
(A) P493-6 cells were synchronized with double thymidine block. Cells were incubated with 2mM thymidine for 12 hours, washed and incubated with normal growth medium for another 12 hours, and underwent a second thymidine treatment for 12 hours. After washing, cells were subsequently treated with either methanol vehicle or MPA and guanosine as indicated. Cells were collected for cell cycle profile analysis at different time points (4 h, 8 h, and 12 h) after release from double thymidine block. Cell cycle profiles were analyzed by flow cytometry using PI staining. Numbers indicate the percentage of the cells in each cell cycle phase (G1/S/G2M). (B) DNA replication and cell cycle profile were examined by BrdU uptake assay. P493-6 cells were pulsed with BrdU for 30 minutes before fixation and subsequent staining. Anti-BrdU FITC antibody was used for measuring the BrdU incorporation. DNA content was assessed with 7-AAD staining. MPA-treated cells were incubated concurrently with the indicated nucleotide (A = 25 µM adenosine; G = 25 µM guanosine; I = 25 µM inosine) supplement for 48 hrs before subjected to the analysis. (C) BrdU uptake assay in Ramos cells treated with MPA or with both MPA and 25 µM guanosine(G). Cells were processed as described in Panel 8B. (D) Immunoblot demonstrating the expression of γ-H2AX, a marker for DNA double strand breaks, in MPA-treated P493-6 cells. Protein lysates from cells with designated conditions were harvested 48 hrs after guanosine treatment. In the groups receiving both MPA and guanosine supplement, the treatment started concurrently. (E) γ−H2AX expression in MPA-treated Ramos cells with or without 25 µM guanosine.