Abstract
Vascular thrombosis is a cause of allograft loss after pancreas transplantation. We present the use of intraoperative fluorescence imaging with the SPY imaging device (Novadaq Technologies Inc, Toronto, Canada) in two pancreas transplants as a means to assess potency of the vascular anastomoses. Intravenous indocyanine green 2.5 mg/mL was fluoresced with the device to create the intraoperative video sequences, which were recorded. After 60-day follow-up, real-time SPY imaging on these two pancreas transplants did not demonstrate adverse effects on patients or the transplanted allografts. This method of vascular imaging could prove useful in improving short-term graft survival and possibly lowering the thrombosis rates seen with pancreas transplantation. Long-term correlation studies between intraoperative findings and graft survival must be performed to confirm the utility of this imaging method.
Pancreas transplantation is now entering its fifth decade with increasing success rates. As reported by Gruessner's review, over 23,000 pancreas transplants have been performed worldwide as of 2004 (1). Immunosuppression management has significantly improved the allograft survival rates. Current regimens include antithymocyte globulin (2) and daclizumab (3) induction with calcineurin inhibitor-based therapy. Technical modifcations in techniques have paralleled the improvements in medications. Thus, significant gains in outcomes and results have been seen.
Technical considerations in the pancreas transplant operation are many. The pancreas is regarded as one of the most unforgiving organs in surgery, and especially in transplant surgery, due to complications related to pancreatitis. Thrombotic complications involving arterial and venous flows in the transplanted allografts comprise over half of the technical complications (4). Additionally, intrinsic perfusion defects due to preexisting microvascu-lopathy of the donor pancreas, injuries sustained in trauma, and iatrogenic vascular injuries to the allograft play a role in immediate and long-term pancreas allograft survival. Pancreas transplants in particular are known to succumb to vascular catastrophes (namely graft thromboses) due to the organ's intrinsic low flow state.
To ensure successful revascularization and allograft perfusion during pancreas transplantation, the Baylor Regional Transplant Institute began using intraoperative fluorescence imaging (IFI) with indocyanine green (ICG). ICG was initially used to determine cardiac output and hepatic function. Intraoperative utilization of ICG has been shown to be extremely beneficial in ophthalmic surgery, and more recently its use in cardiac surgery has been described (5, 6). The imaging can potentially improve the outcomes and economics of cardiac bypass surgery by lowering reparations due to anastigmatic stenoses. Thojimbara and colleagues reported the use of SPY imaging in a renal transplant (7) with multiple renal arteries. Additionally, imaging has been reported in living-donor liver transplantation in order to determine arterial potency and organ perfusion (8).
This preliminary report describes the first two pancreas transplants that were imaged at Baylor Regional Transplant Institute with this method.
MATERIALS AND METHODS
The use of the IFI device in pancreas transplantation was approved by the Baylor University Medical Center institutional review board, and informed consent was obtained from both patients.
Fluorescence imaging/angiography
The SPY imaging system (Novadaq Technologies Inc., Toronto, Canada) comprises an imaging head, containing the optical components, attached to a cart by means of an articulated arm. The cart contains electronics, a computer, and a video monitor (Figure 1). The imaging is based on the fuorescence properties of ICG (IC-Green, Akorn Pharmaceuticals). The dye has an excellent safety profile with very few adverse events, with most such events being minor in severity (9, 10).
Figure 1.

The SPY imaging system. Photo courtesy of Novadaq Technologies.
To acquire images, the imaging head (enclosed in a sterile drape) is positioned over the area of interest at a distance of 12 inches. A bolus of ICG is injected through the central venous line, and the laser/image capture is activated. As the ICG passes through the feld of view, the dye fluoresces and the fluorescent images are captured and displayed on the monitor in real time. The system shuts of after 34 seconds of image acquisition, at which time the images can be reviewed in detail and saved to hard drive. Subsequent image sequences can be acquired within 2 to 3 minutes.
For imaging of the pancreas allografts, 1.0 mL of ICG (2.5 mg/mL) was administered via the central venous line. The ICG was administered as a tight bolus followed by 10 mL of intravenous crystalloid solution “chaser.” Images were acquired upon completion of the vascular anastomoses and reperfusion of the pancreas allograft. In addition to imaging of the vascular anastomoses, images were also acquired to demonstrate perfusion of the whole pancreaticoduodenal allograft.
Operative technique
Pancreas transplantation was done using the technique described by Boggi (11). Intraoperative exploration via midline incision was performed. Full mobilization of the ascending colon to the mid transverse colon was performed. The superior mesenteric vein of the recipient was identified by this approach and provided the venous outflow into the recipient's portal system. The arterial inflow was either the distal aorta or right common iliac artery, depending on the suitability for vessel anastomosis.
The harvested whole pancreaticoduodenal allografts were prepared at the back table under cold conditions. Same-donor iliac “Y” vessel allografts were anastomosed to the donor superior mesenteric artery and splenic artery of the pancreas allograft in an end-to-end fashion. Backtable splenectomy was performed with vascular TA 30 staplers, and control of the divided small bowel mesentery was performed with staples also.
The pancreas allograft was implanted after adequate vascular exposure and delivery of a 3000-U bolus of heparin. The donor portal vein was anastomosed to the superior mesenteric vein in an end-to-side fashion followed by the implantation of the donor arterial conduit to the distal aorta or common iliac in an end-to-side fashion. After hemostasis was obtained, intraoperative imaging was performed on the transplanted whole pancreaticoduodenal allografts.
RESULTS
The pancreas allografts were successfully imaged with the SPY device. The first case was a 36-year-old man with end-stage renal disease (predialysis) and type I diabetes mellitus who received an ABO-compatible pancreas and kidney transplant. The second case (Figure 2) was a 50-year-old man with end-stage renal disease due to type I diabetes mellitus who underwent successful living-related donor renal transplant 2 years before pancreas transplantation. In both cases, 1 mL ICG was required for arterial imaging, 1 mL for venous imaging, and 1 mL for pancreatic perfusion imaging.
Figure 2.
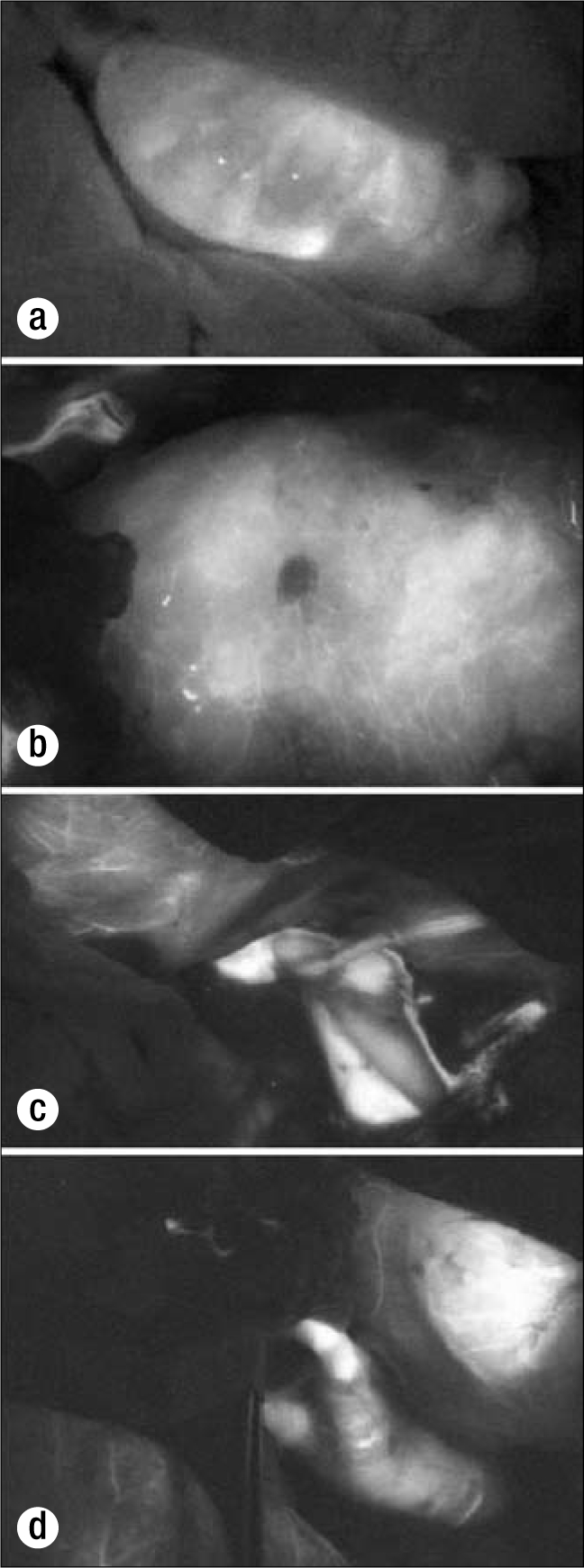
Fluorescence imaging in case 2: (a) the duodenal segment (note that the adjacent small intestine has cleared ICG); (b) the transplanted pancreas body; (c) the portal–superior mesenteric vein anastomosis; (d) the arterial conduit to the pancreas allograft.
During the course of the imaging, the surrounding tissues took up ICG. Most notably, the surrounding colon and small intestine were shown to take up ICG and release it, as with normal perfusion and washout. The transplanted duodenal segment demonstrated prompt uptake but delayed washout. This was seen with the pancreas allograft as well.
We observed what appeared to be slow flow out of the pancreatic allograft through the portal vein in both cases. Additionally, the arterial inflow was imaged (measured to be 100–200 mL/min using electromagnetic flow meter [Clinifow 2000, Braemar Inc, Eagan, MN]), and the time from appearance in the artery to perfusion of the organ was nearly simultaneous. The clearance of the ICG from the pancreas allograft and duodenal segments was in excess of 20 minutes, despite the rapid clearance from the surrounding native intestine.
Clinically, both pancreas allografts had immediate function, maintaining euglycemia, and demonstrated mild to minimal preservation injury based on the serial serum amylase and lipase determinations. The recipient who received a pancreas after kidney transplant was anticoagulated posttransplant according to our institutional protocol. The recipient who received a simultaneous pancreas/kidney transplant was placed on 48 hours of continuous hetastarch infusion, with conversion to aspirin and dipyridamole. No early technical complications were seen in either patient.
Routine Doppler ultrasound imaging posttransplant was performed, and in one case evaluation by standard angiography was done in response to hyperglycemia that persisted until the patient was converted from tacrolimus to cyclosporine.
DISCUSSION
Since vascular thrombotic events following pancreas transplantation are a major cause of graft loss and graft failure, the ability to image allografts intraoperatively to ensure adequate vascularization seems to be an important advance.
The use of ICG and fuorescence angiography with the SPY imaging device has provided insight into the low flow state of the transplanted pancreas allografts. This has not been demonstrated outside of animal models. Additionally, use of this device demonstrated pancreatic and duodenal graft perfusion. The period of time to total washout of the ICG from the transplanted allograft (i.e., allograft retention of the ICG) may be related to significant vasospasm in the distal capillary beds from ischemia-reperfusion phenomena or may be due to low flow intrinsic to the pancreas transplant allograft. We have shown that this method of imaging is tolerated by the transplanted pancreas allograft and its recipient; therefore, further development of this mode of imaging should be performed in order to minimize vascular complications and optimize long-term results. Clinical correlation studies in a controlled manner should be performed to quantify allograft perfusion quality and its impact on short-term allograft function and survival.
References
- 1.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005;19(4):433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 2.Stratta RJ, Sundberg AK, Farney AC, Rohr MS, Hartmann EL, Adams PL. Experience with alternate-day thymoglobulin induction in pancreas transplantation with portal-enteric drainage. Transplant Proc. 2005;37(8):3546–3548. doi: 10.1016/j.transproceed.2005.09.084. [DOI] [PubMed] [Google Scholar]
- 3.Stratta RJ, Alloway RR, Lo A, Hodge EE. A prospective, randomized, multicenter study evaluating the safety and efficacy of two dosing regimens of daclizumab compared to no antibody induction in simultaneous kidney-pancreas transplantation: results at 3 years. Transplant Proc. 2005;37(8):3531–3534. doi: 10.1016/j.transproceed.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 4.Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: why grafts fail and the risk factors—a multivariate analysis. Transplantation. 2004;78(8):1188–1192. doi: 10.1097/01.tp.0000137198.09182.a2. [DOI] [PubMed] [Google Scholar]
- 5.Reuthebuch O, Haussler A, Genoni M, Tavakoli R, Odavic D, Kadner A, Turina M. Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest. 2004;125(2):418–424. doi: 10.1378/chest.125.2.418. [DOI] [PubMed] [Google Scholar]
- 6.Balacumaraswami L, Abu-Omar Y, Choudhary B, Pigott D, Taggart DP. A comparison of transit-time fowmetry and intraoperative fuorescence imaging for assessing coronary artery bypass graft patency. J Thorac Car-diovasc Surg. 2005;130(2):315–320. doi: 10.1016/j.jtcvs.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Sekijima M, Tojimbara T, Sato S, Nakamura M, Kawase T, Kai K, Urashima Y, Nakajima I, Fuchinoue S, Teraoka S. An intraoperative fuorescent imaging system in organ transplantation. Transplant Proc. 2004;36(7):2188–2190. doi: 10.1016/j.transproceed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Kubota K, Kita J, Shimoda M, Rokkaku K, Kato M, Iso Y, Sawada T. Intraoperative assessment of reconstructed vessels in living-donor liver transplantation, using a novel fuorescence imaging technique. J Hepato-biliary Pancreat Surg. 2006;13(2):100–104. doi: 10.1007/s00534-005-1014-z. [DOI] [PubMed] [Google Scholar]
- 9.Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn. 1989;17(4):231–233. doi: 10.1002/ccd.1810170410. [DOI] [PubMed] [Google Scholar]
- 10.Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, So-renson JA, Krupsky S, Orlock DA, Puliafto CA. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101(3):529–533. doi: 10.1016/s0161-6420(94)31303-0. [DOI] [PubMed] [Google Scholar]
- 11.Boggi U, Vistoli F, Signori S, Del Chiaro M, Amorese G, Vanadia Bartolo T, Croce C, Sgambelluri F, Marchetti P, Mosca F. Outcome of 118 pancreas transplants with retroperitoneal portal-enteric drainage. Transplant Proc. 2005;37(6):2648–2650. doi: 10.1016/j.transproceed.2005.06.081. [DOI] [PubMed] [Google Scholar]


