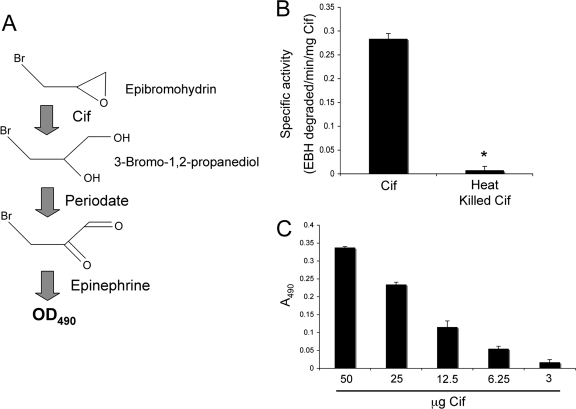
FIG. 1.
Cif degrades the epoxide EBH. The degradation of epoxides by EHs creates highly reactive vicinal diols. The colorimetric detection of these diols is outlined in panel A. Generation of the vicinal diol of EBH by Cif can be detected spectrophotometrically through the oxidation of 3-bromo-1,2-propanediol by periodate and subsequent analysis of the redox state of epinephrine added to the reaction. (B) Purified Cif (50 μg) was incubated with 10 mM EBH, and absorbance at 490 nm was monitored. Also shown is a heat-inactivated protein (heat kill) control. Data were normalized to samples containing buffer alone. *, P value of <0.05. Values are presented as specific activity (EBH degraded/min/mg Cif). (C) Dose response of EBH degradation by Cif. Bar 1 shows 10 mM EBH and 50 μg Cif; bar 2, EBH and 25 μg Cif; bar 3, EBH and 12.5 μg Cif; bar 4, EBH and 6.25 μg Cif; and bar 6, EBH and 3 μg Cif. Samples were incubated at 37°C for 15 min. Values are presented as the OD490, with the OD490 measurements of each condition subtracted from the control condition containing EBH alone.