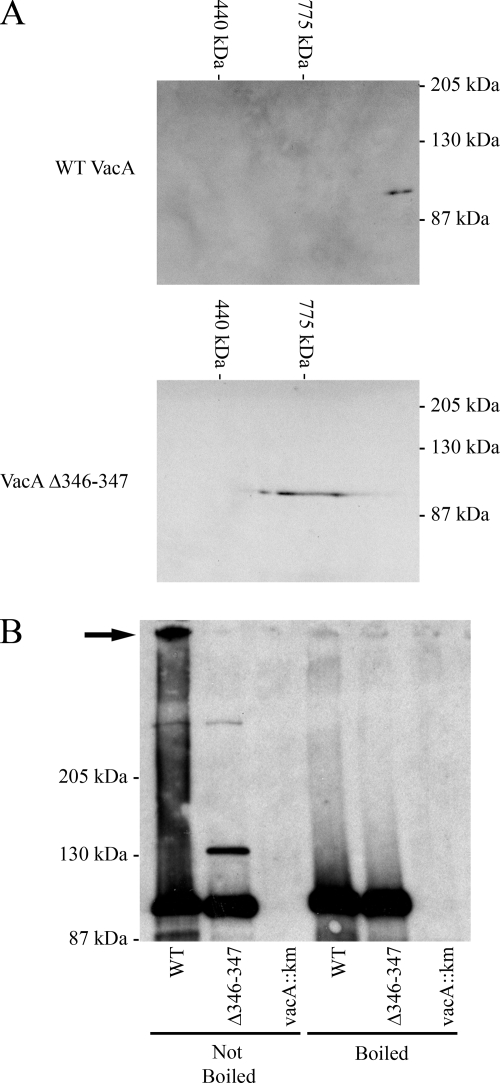
FIG. 3.
Analysis of oligomer formation by wild-type VacA and VacA Δ346-347. (A) BN-PAGE. Wild-type VacA (top panel) and VacA Δ346-347 (bottom panel) were purified and then analyzed by BN-PAGE, followed by immunoblotting with an anti-VacA serum. (B) Analysis by modified SDS-PAGE. VacA was precipitated from H. pylori broth culture supernatants with ammonium sulfate and VacA protein concentrations were normalized based on immunoblot analysis. Equivalent amounts of precipitated proteins were suspended in an SDS-containing buffer. One set of preparations (lanes 1 to 3, left) was not boiled, and a duplicate set of preparations (lanes 4 to 6, right) was boiled prior to SDS-PAGE. Samples were run on a 6% SDS gel, followed by immunoblotting with an anti-VacA serum. The arrow indicates a large oligomeric VacA complex. WT, wild type.