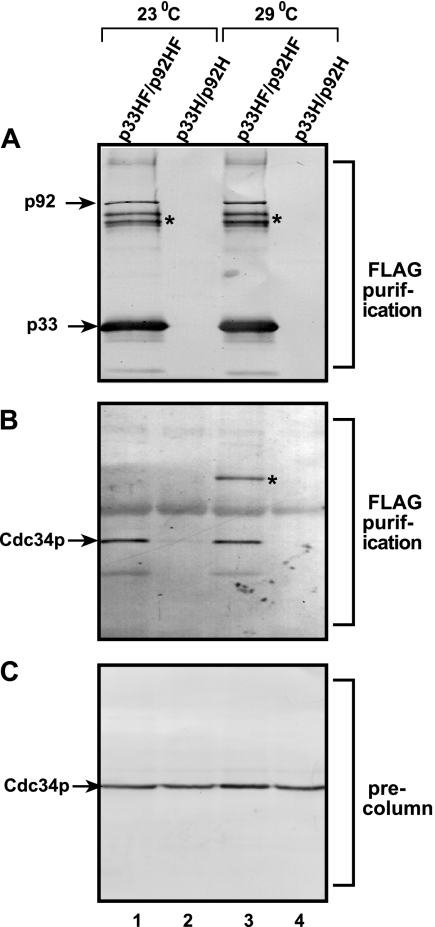
FIG. 6.
Copurification of Cdc34p with the tombusvirus replicase complex. (A) FLAG- and His6-tagged p33 (p33HF) and p92pol (p92HF) replication proteins were purified after detergent-based solubilization of a membrane-enriched fraction of yeast on a FLAG affinity column. Western blotting with anti-His6 antibody detected the presence of p33 and p92pol in the purified replicase complex active in an in vitro replication assay (not shown), as demonstrated by Serva and Nagy (49). The control samples were from yeast expressing His6-tagged p33 (p33H) and p92pol (p92H) prepared as described above for p33HF and p92HF. Asterisks show p33 dimers that are partly resistant to denaturing conditions. (B) Western blotting of the same samples as in panel A with anti-Cdc34p antibody. Note that native Cdc34p was expressed from its original promoter and its original location on the chromosome. Occasionally, the Cdc34p formed a dimer when the yeast was grown at 29°C (marked with an asterisk in lane 3). (C) Western blotting analysis with anti-Cdc34p antibody showing similar amounts of Cdc34p present in the membrane-enriched fractions prior to affinity purification.