FIG. 2.
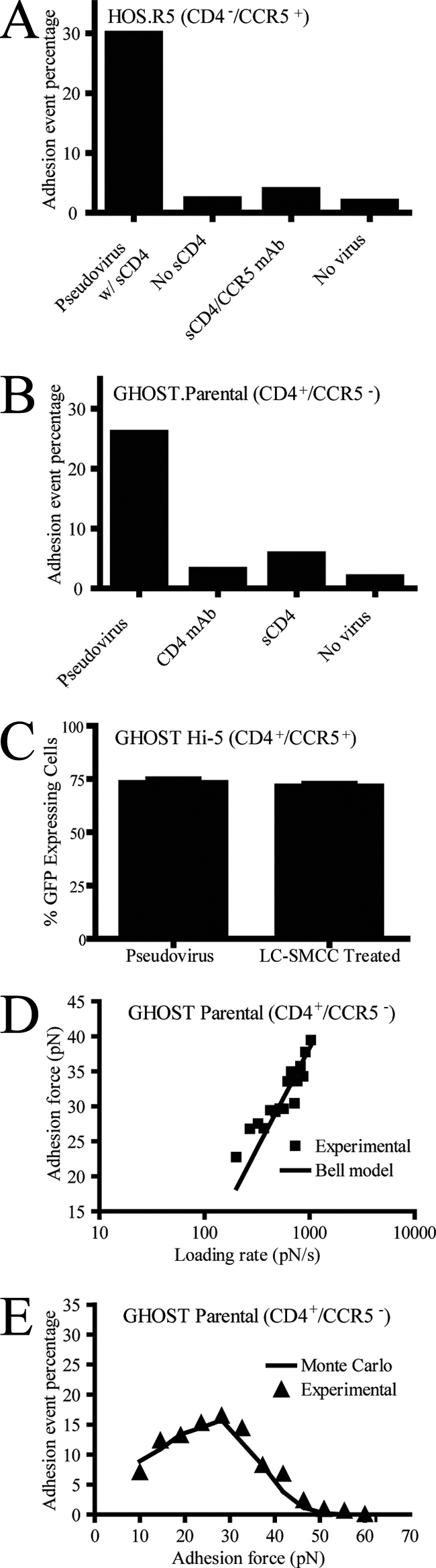
Test of binding specificity and characterization of virion-receptor interactions at single-molecule resolution. (A) Test of specificity of MFP measurements and frequency of binding interactions between Env glycoproteins and CD4− CCR5+ living cells in the presence of sCD4, in the absence of sCD4, in the presence of a function-blocking antibody against CCR5 (CCR5 mAb), or in the absence of virions attached to the cantilever (No virus), respectively. (B) Test of specificity of MFP measurements and frequency of binding interactions between Env glycoproteins and CD4− CCR5+ living cells in the absence of added molecules, in the presence of a function-blocking antibody against CD4 (CD4 mAb; B4), in the presence of sCD4, or in the absence of virions (No virus), respectively. (C) Comparison of CD4+ CCR5+ cells infected to express GFP with pseudotyped virus with and without LC-SMCC treatment. (D) Mean adhesion force of the gp120-CD4 bond as a function of loading rate (pN/s) for CD4+ CCR5− parental cells. Fit of this curve using Bell's model yielded a bond dissociation constant, koff0, of 3.73 s−1 and a bond reactive compliance, xβ, of 0.34 nm. (E) Distribution of adhesion bond forces obtained experimentally (triangles) or computed using a Monte Carlo simulation (line) based on Bell model's kinetic parameters (see text for details). The retraction velocity of the cantilever was maintained at 10 μm s−1.
