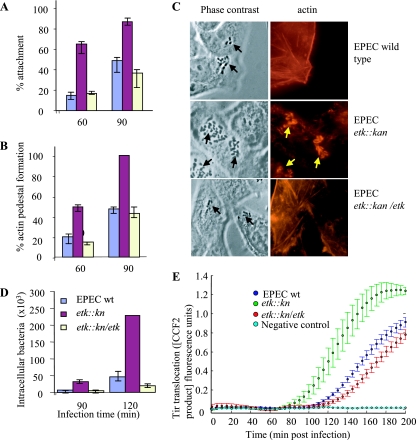
FIG. 2.
The G4C interferes with EPEC interaction with host cells at early time points postinfection. HeLa cells were infected with overnight cultures (multiplicity of infection, ∼2) of wild-type (wt) or etk::kan mutant EPEC or with the mutant complemented with an Etk-expressing plasmid (etk::kan/etk). At different time points postinfection, cells were washed, fixed, and stained with phalloidin-rhodamine, and the attachment (A) and pedestal formation (B) levels were determined. Bacterial adhesion was evaluated as the percentage of cells with eight or more bacteria attached out of the total number of cells in the microscopic field. The mean percentages and standard deviations of 10 random microscopic fields (∼200 cells) are shown. The efficiency of actin pedestal formation was evaluated as the fraction of attached bacteria forming pedestals out of the total number of attached bacteria, and the mean percentages and standard deviations of five random microscopic fields (∼120 HeLa cells) are shown. Typical phase-contrast and corresponding actin-stained (red) images taken at 60 min postinfection are shown in panel C. The black arrows indicate bacteria, and the yellow arrows indicate actin pedestals. In a different experiment, cells were infected with similar overnight cultures and the invasiveness of the different strains at early time points postinfection was compared by using the gentamicin protection assay (D). The assay was carried out in triplicate as previously described (4), and standard deviations are shown by bars. In panel E, the same mutants, supplemented with the reporter tirEPEC-blaM gene, were used to infect HeLa cells preloaded with CCF2. The kinetics of translocation by the three mutants were determined in parallel and in real time as previously described (30). Each time point represents the average translocation by six different colonies, and the bars indicate standard errors.