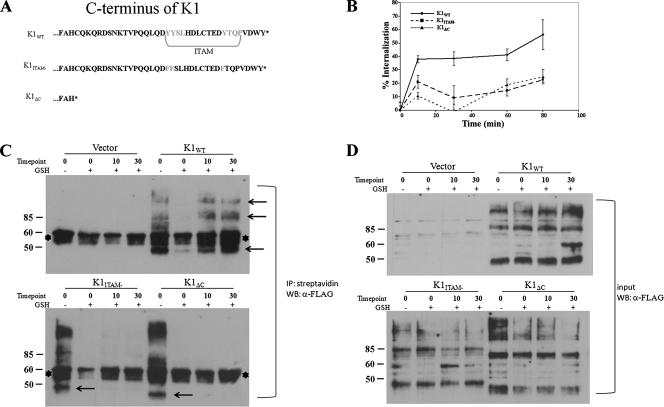
FIG. 1.
Internalization of K1 in HeLa cells. (A) Diagram of the amino acids in the C termini of K1 and mutants. The ITAM is highlighted. (B) Transfected cells were surface labeled with M1 anti-FLAG antibody at 4°C for 1 h, washed, and incubated for the times indicated at 37°C. Antibody remaining on the surface was stripped off, and the cells were lysed. Internalized antibody was quantified by ELISA. The data are expressed as a percentage of the initial amount of antibody bound to the cell surface at 0 min at 4°C. The error bars indicate standard deviations. (C) Endocytosis of K1 in a biotin endocytosis assay. HeLa cells were transfected with K1 and mutant expression plasmids as indicated above each gel. The samples were processed for the biotinylation endocytosis assay as described in Materials and Methods. The biotinylated proteins were internalized by transferring the cells to 37°C for 10 and 30 min. The + and − symbols indicate presence and absence of glutathione (GSH) treatment. The cells were then lysed, and all biotinylated proteins were immunoprecipitated (IP) using streptavidin beads. FLAG-K1 was detected in cell surface and intracellular pools by immunoblotting. The arrows indicate K1-specific bands. The asterisks denote nonspecific bands. (D) The same samples as in panel C, representing 1% of the input sample before immunoprecipitation.