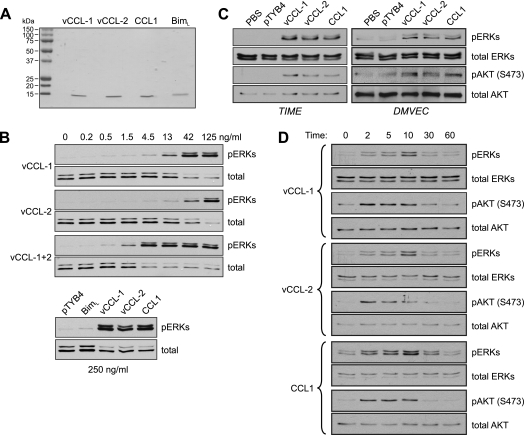
FIG. 1.
Activation of AKT and ERK1/2 by viral and cellular CCR8 agonists in endothelial cells. (A) Recombinant vCCL-1, vCCL-2, and CCL1, along with Bim (negative control), were generated as described in Materials and Methods, and their integrity and purity were checked on Coomassie-stained gels. (B) Viral chemokine activities and signaling in endothelial cells were checked in ERK activation assays in TIME cells. Cells were serum and supplement deprived for 24 h and then treated with different doses of the v-chemokines, alone or combined, for 10 min prior to cell harvest for analysis of ERK activation. Phospho-specific antibody was used to identify threonine-202/tyrosine-204-phosphorylated ERKs (pERKs) by Western analysis. Antibodies recognizing ERKs independently of phosphorylation status were used to confirm equal protein loading and transfer. In a parallel experiment, cultures were treated with 250 ng of the v-chemokines, CCR1, or BimL (nonsignaling control), or with pTYB4 (empty bacterial expression vector) transformant extract processed identically and in parallel with the recombinant protein-containing samples. (C) ERK and AKT activation assays were undertaken in TIME cells and primary DMVEC treated with maximally effective doses (250 ng/ml) of recombinant vCCL-1, vCCL-2, or CCL1. Antibody recognizing serine-473-phosphorylated AKT (pAKT) was employed to identify the active form of the kinsase by immunoblotting. (D) Similar experiments were undertaken in TIME cells, which were harvested at different times poststimulation to determine the kinetics and durations of ERK and AKT activation.