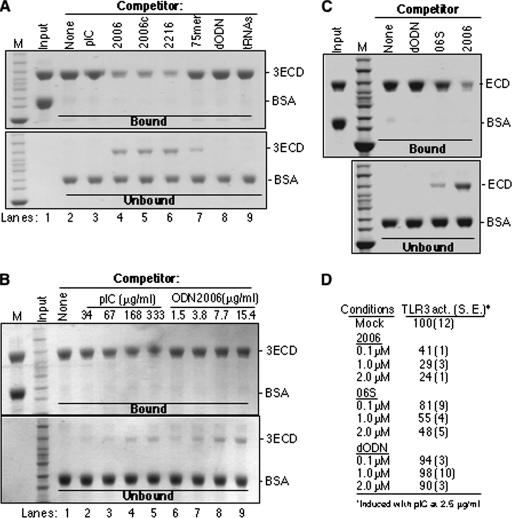
FIG. 8.
ODN2006 is preferentially bound by TLR3. (A) An affinity pull-down assay was used to compare the binding of various ligands to 3ECD. The input used was a mixture of purified 3ECD and BSA. The mixture was then incubated with washed ODN2006-streptavidin resin in the presence of the competitor nucleic acid shown above the gel image. All competitors were used at 3 μg in the binding reaction mixtures. The gels were stained with Coomassie blue. Proteins bound and not bound to the resin are shown in the upper and lower gel images. The identities of the bands are shown to the right of the gel images. The binding reactions were performed at room temperature. (B) A comparison of the ability of heterogeneous poly(I-C) to compete with ODN2006 for binding of 3ECD. All concentrations are shown as μg/ml to facilitate comparisons. (C) ssDNA 06S that is partially defective for inhibiting TLR3 signaling is also defective for preventing 3ECD binding to ODN2006-resin. All competitors were added to the binding reaction mixture at 2 μM. (D) Results of reporter assay demonstrating that 06S is defective for inhibiting TLR3 signaling. The results are from the standard NF-κβ reporter assay of HEK 293T cells transfected to express TLR3. The cells were induced with poly(I-C) in the presence of the oligonucleotides at the concentrations shown. 2006, 2006c, and 2216 are ODNs. M, size marker; pIC, poly(I-C); act., activity; S.E., standard error.