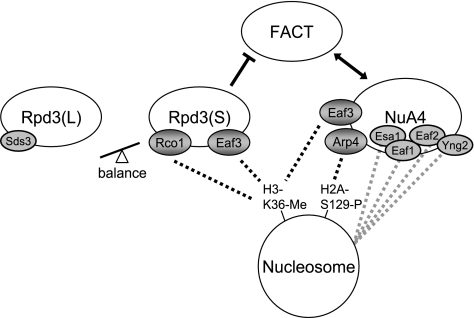
FIG. 10.
Model of competition between Rpd3(S) and NuA4. Rpd3(S) and NuA4 each have at least two subunits that mediate association with nucleosomes. Rco1 and Eaf3 each recognize methylated K36 of histone H3. Arp4 is thought to recognize histones in several ways, one of which is dependent on phosphorylation of H2A(S129). FACT NuA4 double mutants show synthetic phenotypes, and a mutation in one reduces binding of the other factor, and thus, NuA4 and FACT each reinforce the function of the other factor. Mutations which decrease Rpd3(S) binding to nucleosomes increase NuA4 binding and also stimulate FACT activity. The balance between the two types of Rpd3 HDAC complexes is important, and changing this balance can either suppress or exacerbate FACT mutant phenotypes.