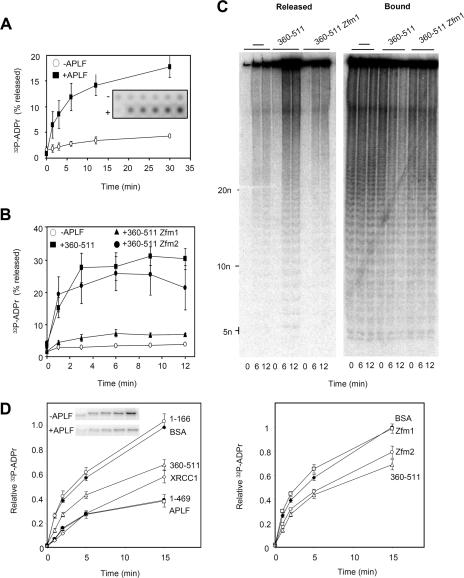
FIG. 4.
Impact of APLF on pADPr metabolism in vitro. (A) Release of [32P]pADPr from ribosylated histone H1.2 by APLF. Multiwell dishes coated with recombinant human histone H1.2 were poly(ADP-ribosyl)ated in the presence of NAD+ that was spiked with 32P-NAD+ and then washed and incubated with His-APLF (+APLF) or buffer alone (−APLF) for the times indicated at 30°C. Released material and bound material were detected by phosphorimager, and the released material plotted as a percentage of total 32P signal (released plus bound) in each reaction mixture. Inset shows a representative phosphorimage. (B) Release of [32P]pADPr from ribosylated histone H1.2 by the C-terminal domain of APLF. Ribosylated histone H1.2 was incubated as described above with buffer alone, His-APLF360-511, His-APLF360-511zfm1, or His-APLF360-511zfm2 for the times indicated. Other details are as described for panel A. (C) Analysis by native PAGE of material generated in one of the experiment whose results are shown in panel B. −, buffer alone. (D) The results in the left panel show the impact of APLF on PARP-1 autoribosylation. PARP-1 autoribosylation was conducted in the presence of NAD+ that was spiked with 32P-NAD+, and 32P-labeled PARP-1 was quantified on acid PAGE gels by phosphorimager. Reaction mixtures included BSA, His-APLF, His-APLF360-511, His-APLF1-166, His-APLF1-469, or His-XRCC1. A representative phosphorimage showing PARP-1 ribosylation is included (inset). Data are plotted relative to the signal obtained after 15 min of incubation in the presence of BSA (negative control). The results in the right panel show the impact of the C-terminal domain of APLF on PARP-1 autoribosylation. Reactions were conducted as described for the left panel, and the reaction mixtures included BSA, His-APLF360-511, His-APLF360-511zfm1, or His-APLF360-511zfm2. Error bars show standard errors of the mean.