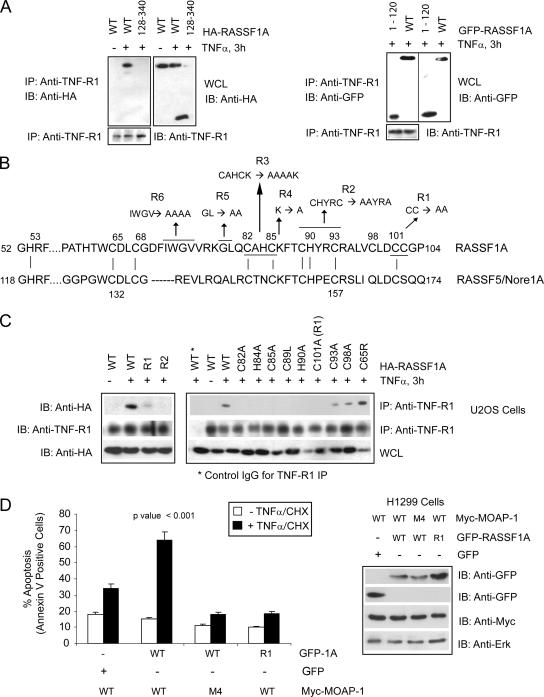
FIG. 6.
Location of RASSF1A residues important for TNF-R1 association. (A) Coimmunoprecipitation (IP) of endogenous TNF-R1 with wild-type (WT) HA-RASSF1A and a deletion mutant lacking the N terminus of RASSF1A (left panel, 128-340) or C terminus (right panel, 1-120). Numbers indicate amino acid locations. (B) Amino acid sequence of the C1 domain of RASSF1A and RASSF5/Nore1A with indicated mutants of RASSF1A (R1, R2, etc.). Vertical lines between the two protein sequences indicate locations of conserved cysteine and histidine residues involved in the formation of the coordination spheres for zinc (as characterized for RASSF5/Nore1A by Harjes et al. [19]) Numbers indicate amino acid locations, and underlined residues and arrows indicate amino acid changes to alanine with the RASSF1A sequence. (C) Coimmunoprecipitation of endogenous TNF-R1 with HA-RASSF1A wild type or point mutants within the C1 domain of RASSF1A (R1 and R2 and indicated mutations). Numbers indicate amino acid location changes to alanine within the RASSF1A sequence. IgG, immunoglobulin G; L, leucine; R, arginine; A, alanine. (D) The left panel shows annexin V staining of TNF-α-CHX-treated H1299 cells stably expressing the Myc-MOAP-1 wild type or MOAP-1 mutant M4 (Fig. 4A). GFP constructs were transiently transfected to the level of 30 to 40%. Experiments were repeated six times, and significance was evaluated by one-way analysis of variance (P < 0.001). The right panel shows expression of proteins used in the annexin V staining experiment.