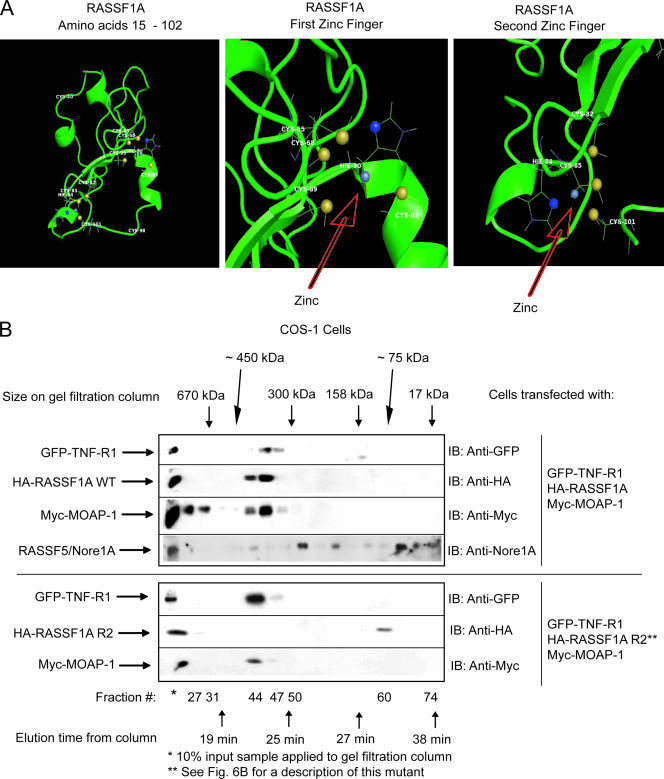
FIG. 7.
Ternary complex formation between RASSF1A and TNF-R1 requires an intact C1 domain of RASSF1A. (A) Computer-generated model of residues 15 to 102 of RASSF1A. This model was generated by sequence and structural comparison with the NMR structure of RASSF5/Nore1A using the MODELLER program (38) and ROSETTA comparative modeling protocol (9, 27). The left panel shows the structure of residues 15 to 102 of RASSF1A, revealing two zinc finger motifs as indicated. The middle panel shows the first zinc finger motif with the zinc coordinated to four residues (C-68, C-89, H-90, and C-93). The right panel shows the second zinc finger motif with the zinc coordinated to four residues (C-82, H-84, C-85, and C-101). The zinc atom is gray, the histidine ND1 atoms are blue, and the cysteine SG atoms are yellow. (B) Transfection was carried out (as indicated) on a 4- by 10-cm2 dish using 5 μg of each component. (See Fig. 6B for a description of RASSF1A R2, a RASSF1A mutant that lacks the ability to associate with TNF-R1.) Plate samples were pooled by scraping into 1 ml of 1× PBS, followed by lysis in 800 μl of RIPA buffer. Pooled samples were then analyzed by gel filtration analysis as described in the legend to Fig. 2. Fractions were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. IB, immunoblotting. This experiment was carried out two times with similar outcomes. A representative experiment is shown.