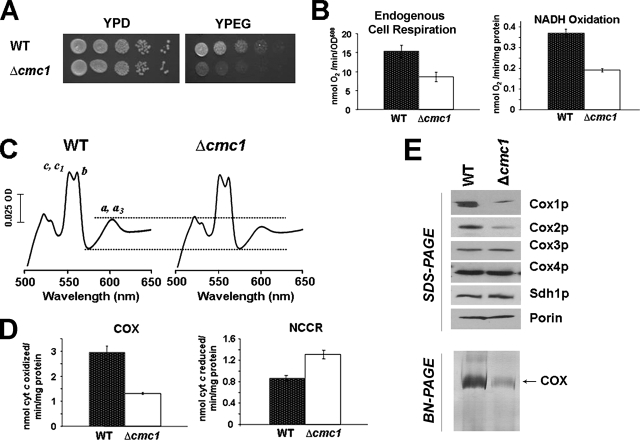
FIG. 1.
Characterization of a cmc1 null mutant. (A) The respiration-competent wild-type strain BY4741 (WT) and a strain carrying a null allele of cmc1 (Δcmc1) were grown overnight in liquid YPD medium. Tenfold serial dilutions of the two strains were plated on solid YPD or YPEG medium and incubated at 30°C. Pictures were taken after 3 days of incubation. (B) Respiratory properties of cmc1 mutant cells. KCN-sensitive endogenous cell respiration was measured polarographically in the presence of galactose. Oxygen consumption was also assayed in mitochondria prepared from the BY4741 wild-type strain and the Δcmc1 null mutant in the presence of NADH as the substrate as described in Materials and Methods. The bars indicate the means ± SD from at least three independent sets of measurements. (C) Total mitochondrial cytochrome spectra. Mitochondria from the wild-type strain BY4741 and the Δcmc1 null mutant were extracted at a protein concentration of 5 mg/ml with potassium deoxycholate under conditions that quantitatively solubilize all the cytochromes (39). Difference spectra of the reduced (sodium dithionite) versus oxidized (potassium ferricyanide) extracts were recorded at room temperature. The absorption bands corresponding to cytochromes a and a3 have maxima at 603 nm; the maxima for cytochrome b and for cytochromes c and c1 are 560 and 550 nm, respectively. (D) Mitochondrial respiratory chain enzyme spectrophotometric measurements in isolated mitochondria. COX and NADH cytochrome c reductase (NCCR) were measured as described in Materials and Methods. (E) Steady-state concentrations of COX subunits estimated by Western blot analyses of proteins separated by 12% Tris-glycine SDS-PAGE are shown in the upper panel. In the lower panel, extracts of 100 μg of mitochondrial proteins, prepared as described in Materials and Methods, were loaded on a BN-polyacrylamide gel. The gel was stained for COX activity. With the conditions used, only the monomeric COX is detected.