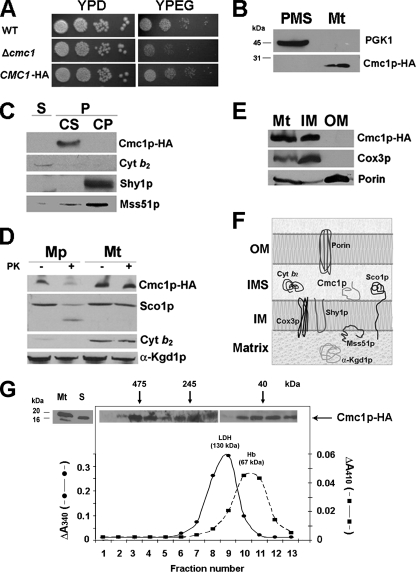
FIG. 2.
Mitochondrial localization of Cmc1p-HA. (A) Expression of an HA-tagged version of Cmc1p complements the respiratory growth defect of Δcmc1 mutant. The respiration-competent wild-type strain BY4741 (WT), a strain carrying a null allele of cmc1 (Δcmc1), and the mutant strain with a plasmid expressing HA-tagged Cmc1p (CMC1-HA) were grown overnight in liquid YPD medium. Tenfold serial dilutions of the two strains were plated on solid YPD or YPEG medium and incubated at 30°C. Pictures were taken after 3 days of incubation. (B) Cmc1p-HA is a mitochondrial protein. Mitochondria (Mt) and the postmitochondrial supernatant (PMS) fraction were isolated from the CMC1-HA strain. Samples of the two fractions corresponding to 40 μg of protein were separated by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with antibodies against HA (Cmc1p-HA) or the cytosolic marker 3-phosphoglycerate kinase subunit 1 (Pgk1p). (C) Cmc1p-HA is an extrinsic membrane protein. Soluble (S) and membrane-bound (P) mitochondrial proteins were separated from 40 μg of total mitochondria by brief gentle sonication followed by centrifugation at 35,000 rpm for 30 min. at 4°C. The pellet (P) was resuspended in 0.6 M sorbitol-20 mM HEPES buffer containing 0.1 M Na2CO3 [pH 11] and 50 mM EDTA and incubated on ice for 30 min. Centrifugation at 35,000 rpm for 30 min at 4°C allows the separation of the extrinsic proteins present in the supernatant (CS) from the intrinsic proteins in the pellet (CP). The different samples were loaded on a 10% Tris-Tricine gel, and Western blotting was performed against cytochrome b2, HA (Cmc1p-HA), Shy1p, and Mss51p. (D) Cmc1p-HA is a membrane protein facing the IMS. Four aliquots of 40 μg of mitochondrial protein were pelleted and resuspended in buffer containing either 20 mM HEPES or 0.6 M sorbitol-20 mM HEPES. One aliquot in each buffer was supplemented with final concentration of 100 μg/ml proteinase K (PK) and incubated on ice for 60 min. The reaction was stopped with 2 mM phenylmethylsulfonyl fluoride. Mitochondria (Mt) and mitoplasts (Mp) were recovered by centrifugation at 40,000 rpm for 15 min at 4°C. The pelleted fraction was resuspended in gel buffer and loaded on a 10% Tris-Tricine gel. Western blots were probed with antibodies against Cmc1p-HA, Sco1, porin, cytochrome b2, and α-ketoglutarate dehydrogenase. (E) Cmc1p is an inner mitochondrial membrane protein. Isolated mitochondria were fractionated into inner and outer membranes by sonication plus sucrose gradient sedimentation as described previously (24). Whole mitochondria (M), inner membranes (IM), and outer membranes (OM) were loaded on a 10% Tris-Tricine gel, and the levels of porin (outer membrane marker), Cox3p (inner membrane marker), and Cmc1p-HA were detected by immunoblotting. (F) Cartoon depicting the submitochondrial localization and topology of Cmc1p and other mitochondrial proteins used as references. (G) Sedimentation properties of Cmc1p-HA in a linear 7 to 20% sucrose gradient were analyzed as explained in Materials and Methods. The gradient was calibrated with hemoglobin (Hb) (67 kDa) and lactate dehydrogenase (LDH) (130 kDa). Following centrifugation, the gradient was collected in 13 equal fractions. Each fraction was assayed for Hb by absorption at 410 nm and for LDH activity by measuring NADH-dependent conversion of pyruvate to lactate. The distribution of Cmc1p was assayed by Western blot analysis. The mass of Cmc1p was determined from the positions of the respective peaks relative to those of the markers.