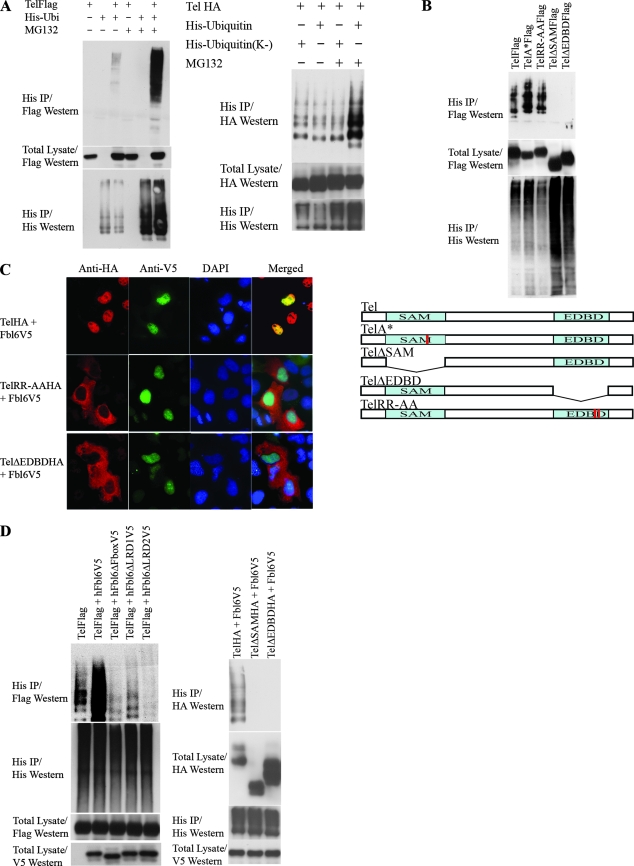
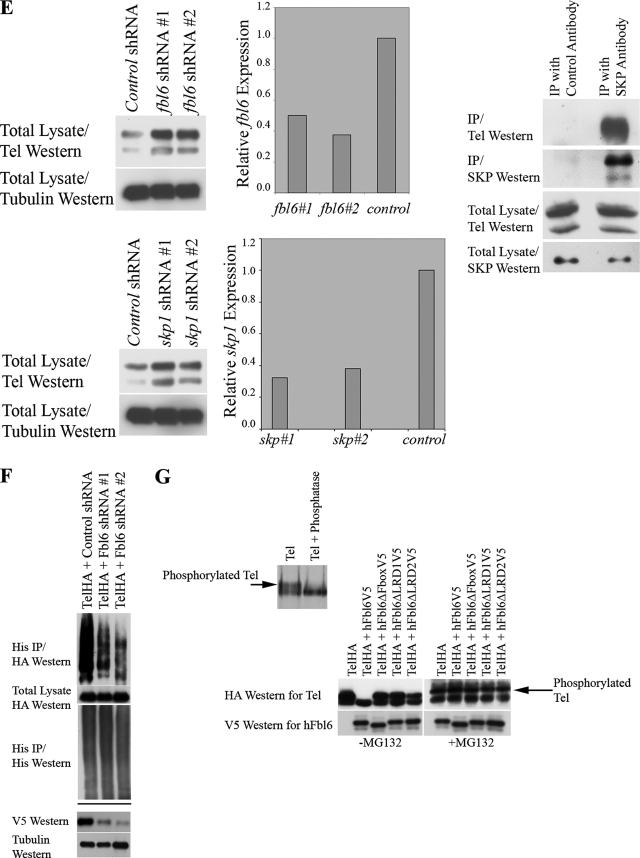
FIG. 2.
(A) Tel ubiquitination. The left panel shows the results of an experiment in which Flag epitope-tagged wild-type Tel was cotransfected into U2OS cells with (+) or without (−) His epitope-tagged ubiquitin and cells were subsequently incubated in the presence (+) or absence (−) of MG132. Ubiquitinated Tel was recovered from cells lysed in guanidinium, by nickel bead purification (hereafter termed a ubiquitination assay). The right panel shows a ubiquitination assay using HA epitope-tagged Tel and either a wild-type His epitope-tagged ubiquitin or a His epitope-tagged version of ubiquitin in which all of the lysine residues have been mutated (K−). (B) The SAM domain and EDBD of Tel are required for its ubiquitination. U2OS cells were transfected with the indicated constructs (described in the legend to Fig. 1B), and a ubiquitination assay was performed as described for panel A. (C) Mutations that disrupt the EDBD of Tel lead to the mislocalization of Tel to the cytoplasm. Cells were transfected with the indicated constructs, and immunohistochemistry was performed with the antibodies shown. (D to G) Fbl6 promotes the ubiquitination of Tel. (D) U2OS cells were transfected with the indicated constructs (see the legend to Fig. 1C), and a ubiquitination assay was performed as described for panel A. (E) shRNA-mediated knockdown of fbl6 and skp1 leads to stabilization of endogenous steady-state Tel protein levels. U2OS cells were infected with lentiviruses delivering the indicated shRNAs, and following 2 days of puromycin selection, stably infected cells were lysed and proteins levels monitored with the indicated antibodies. Tubulin was used as a loading control for proteins. Quantitative PCR was used to demonstrate effective knockdown of fbl6 and skp1 transcripts. In both cases, quantitative PCR was also used to show that tel transcript levels were unaffected by any of the shRNA treatments (data not shown). The rightmost panel shows that endogenous Tel and endogenous SKP1 form a complex in tissue culture cells. U2OS cells were lysed in protein lysis buffer (see Materials and Methods), and SKP1 was purified from the lysate with a SKP1-specific antibody. Associated endogenous Tel was detected with a Tel-specific antibody. (F) Short-hairpin RNA (shRNA)-mediated knockdown of Fbl6 inhibits Tel ubiquitination. Cells were transiently transfected with the indicated constructs, and a ubiquitination assay was performed 2 days later. In the absence of an effective antibody specific for human Fbl6, the efficiency of Fbl6 knockdown was assessed separately by targeting V5 epitope-tagged Fbl6 expressed in U2OS cells. A nonspecific small interfering RNA was used as a control (see lower panel). (G) Fbl6 strongly promotes the degradation of phosphorylated Tel. The upper panel shows [35S]methionine-labeled Tel that was immunopurified from U2OS cells, following 3 h of labeling, and then treated with 10 U of calf intestinal phosphatase or left untreated for 30 min at 37°C. In the lower panel, cells were transfected with the indicated constructs, incubated in the presence (+) or absence (−) of MG132, and then lysed in Laemmli sample buffer. Proteins were detected with the indicated antibodies. Western, Western blotting; IP, immunoprecipitation; h, human.