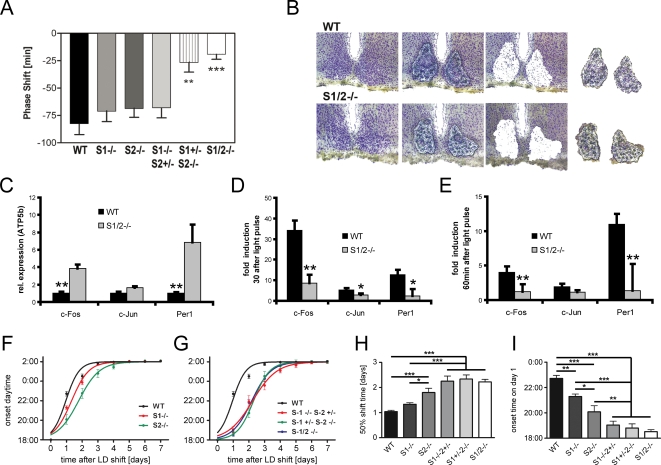
Figure 2. Disturbed phase shifting and clockwork resetting.
(a) Acute activity phase shifts determined for WT and Sharp mutant mice upon a single 15 min light pulse given at ZT14 following the Aschoff type II protocol. S1−/− (71 min±7), S2−/− (69 min±6) and S1−/S2 +/− mutants (68 min±9) did not show a significant phase shift change compared to WT animals (82 min±10). S1+−/S2 −/− (27 min±9, p<0.01 = **) and S1−/S2 −/− (19 min±5, p<0.001 = ***) mutant mice showed a highly significant reduction in phase shift when compared to WT animals (Data are given as mean values ±SEM; n = 12 for WT, S1−/−, S2−/− and S1/2−/−; n = 6 for S1−/−S2+/− and S1+/− S2−/−). (b) Composite picture showing the steps of laser-mediated microdissection of representative WT and S1/2−/− thionin-stained coronal brain cryosections for collection of SCN tissue with high precision. Left panel, the thionin-staining allows for the detection of the SCN by light microscopy; 2nd left panel, micrographs showing the regions selected for isolation; 3rd left panel, sections after isolation; right panels, micrographs obtained from the caps that were used for isolation. (c–e) qRT-PCR data monitoring the relative c-Fos, c-Jun and Per1 expression in the SCN of WT and Sharp mutant mice. Tissue was harvested at ZT14 from control animals without light pulse (c) and 30 min (d) as well as 60 min (d) after 15 min light exposure at ZT14. (c) At ZT14, c-Fos and Per1 expression levels are significantly elevated and c-Jun concentration is slightly increased in the SCN harvested from S1/2−/− mice. (d,e) Photic induction of immediate early and Per1 genes is highly reduced in S1/2−/− mice both 30 (D) and 60 min (E) after the light exposure (* = p<0.05, ** = p<0.01) (Data represent mean values ±SD, n = 3). (f–i) Re-entrainment of WT, S1−/−, S2−/−, S1−/−S2+−, S1+/−S2−/− and S1−/−S2−/− mutant mice to an 8 h delayed LD cycle, respectively. (f) Sharp single mutants display a significantly delayed resetting to the delayed LD cycle (ANOVA for interaction of factors ‘genotypes×time’ WT vs S1−/−: F(7,12.2) = 8.4, p<0.0005; WT vs. S2−/−: F(7,36.0) = 12.4, p<0.0005). Sharp-2 mutants appear to adapt slower compared to Sharp-1 mutants in this task. (g) The loss of additional Sharp alleles further delays the re-adaptation kinetics compared to the WT and single mutants in the 8 h phase delay paradigm. ANOVA for interaction of factors ‘genotypes×time’ WT vs S1−/−S2+−: F(7,50.2) = 23.6, p<0.0005; WT vs S1+/−S2−/− F(7,73.6) = 34.6, p<0.0005; S1−/−S2−/−: F(7,13.4) = 3.1, p<0.005). (h) Comparison of the half-maximal (50%) re-adaptation time in days of WT, S1−/−, S2−/−, S1−/−S2+−, S1+/−S2−/− and S1−/−S2−/− mutant mice reveals significant differences between WT and all other genotypes except Sharp-1 mutants (* = p<0.05, ** = p<0.01, *** = p<0.001). (i) The onset time of running wheel activity at the first day after the LD shift reveals significant differences between WT and all genotypes. (Data are given as mean values ±SEM; n = 12 for WT, S1−/−, S2−/− and S1/2−/−; n = 6 for S1−/−S2+/− and S1+/− S2−/−; * = p<0.05, ** = p<0.01, *** = p<0.001).