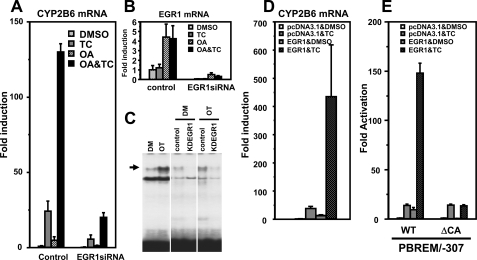
FIGURE 5.
EGR1 required for CAR to activate the CYP2B6 promoter. A–C, siRNA knockdown to show that EGR1 is required for expression of the CYP2B6 gene. Ym17 cells were transfected with EGR1 siRNA or control scramble siRNA and were treated with Me2SO (DMSO), 250 nm TCPOBOP (TC), 10 nm OA, or TCPOBOP plus OA (OA&TC). Total cellular RNAs were prepared from these cells and were subjected to quantitative real time RT-PCR for the CYP2B6 (A) and EGR1 (B) mRNAs. C, gel shift assays to show decrease of EGR1 protein by siRNA. Nuclear extracts were prepared from Ym17 cells transfected with control siRNA (control) and EGR1 siRNA (KDEGR1) and were subjected to gel shift assays using CA-rich probe (–253/–242). The arrows indicate a specific shifted band. D, RT-PCR to demonstrate EGR1 synergizing TCPOBOP activation of the CYP2B6 gene. Ym17 cells were transfected with pcDNA3.1-EGR1 plasmid or empty plasmid (pcDNA3.1) and were treated with Me2SO (DMSO) or TCPOBOP. Total cellular RNAs were prepared from these cells and were subjected to quantitative real time RT-PCR of CYP2B6 mRNA. The levels of CYP2B6 mRNA were normalized by β-actin mRNA levels. -Fold induction was calculated relative to the levels in pcDNA3.1-transfected and Me2SO-treated cells. E, transient transfections to show that EGR1 is required for the CA-rich region to activate the CYP2B6 promoter. Ym17 cells were co-transfected by PBREM/–307 (WT) or its CA-rich deletion mutant (ΔCA) with phRL-SV40, pcDNA3.1 or pcDNA3.1-EGR1. Luciferase reporter activity was measured, and -fold activation was calculated by taking the control activity (pcDNA3.1 with Me2SO) as 1.