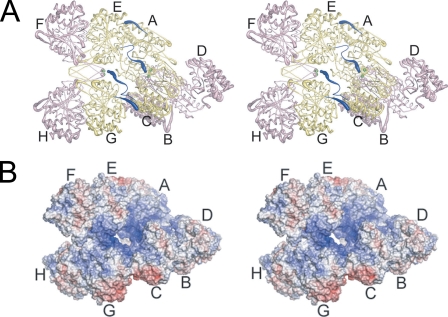
FIGURE 3.
The yeast IDH hetero-octamer does not obey pseudo-222 symmetry. Regulatory IDH1 subunits are shown in yellow and catalytic IDH2 subunits in pink. The N termini of IDH1 subunits are shown in dark blue, and the Sγ atoms of IDH2 Cys-150 residues are shown as bright green spheres. The orientation of the leftmost heterotetramer in each panel is in the same orientation as in Fig. 1C. The thickness of the tubes is proportional to the thermal parameters for the backbone atoms, with thicker tubes representing higher atomic displacement parameters. A, the yeast IDH hetero-octamer with citrate bound. Note that the N termini of two IDH1 subunits run along the interior of the central solvent-filled cavity of the hetero-octamer and the remaining two IDH1 N termini are positioned on the exterior of the hetero-octamer. The interior IDH1 N termini from each heterotetramer interact reciprocally with the Cys-150 residues of theβ-barrel clasp of the other heterotetramer in the ligand-bound but not in the unliganded IDH structures (see “Results”). The 2-fold axis of rotation relating heterotetramers runs normal to a line running from ∼1:00 to 7:00 o'clock in the plane of the page. B, electrostatic surface potential calculated at ±10 kT for the citrate-bound yeast IDH hetero-octamer. The orientation is the same as shown in panel A and in Fig. 1C. Basic amino acid side chains coming from the uncapped IDH1 helices consisting of residues 156-173 and the proximity of the N-terminal amino group of IDH1 chain C and E contribute to a substantial positive electrostatic potential (blue shading) at IDH2 residues Cys-150 (see “Results”).