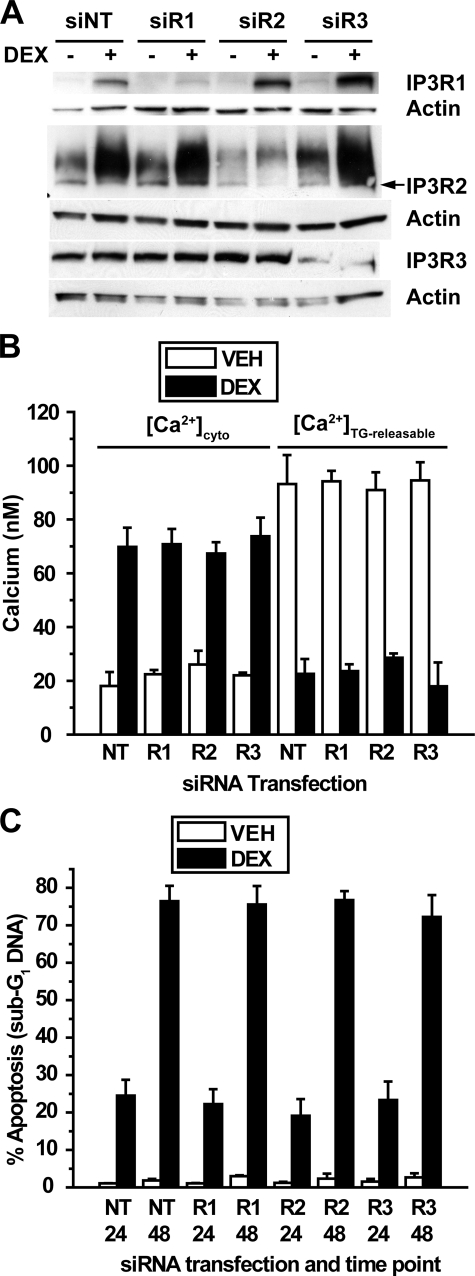
FIGURE 6.
siRNA-mediated repression of individual IP3R isoforms. A, WEHI7.2 cells were transfected with siRNA SMARTpools for IP3R1 (siR1), IP3R2 (siR2), and IP3R3 (siR3) or a non-targeting siRNA SMARTpool (siNT) as a negative control. Cells were treated for 24 h with 100 nm dexamethasone (DEX) or ethanol vehicle. Immunoblots show subtype-specific repression of each subtype. The correct molecular mass band for IP3R2 (∼300 kDa) is the lower, sharper band, as indicated by the arrow. Findings are representative of nine experiments. B, cytosolic calcium and TG-releasable calcium were quantified in cells transfected with SMARTpools for each subtype individually and treated for 24 h with 1 μm dexamethasone or ethanol vehicle (VEH). Error bars represent the mean ± S.E. of three experiments. Each comparison of cytosolic calcium elevation and decreased TG-releasable calcium between dexamethasone-treated and untreated cells was significant (p < 0.01). For dexamethasone-treated cells, each comparison of cytosolic calcium elevation and decreased TG-releasable calcium between non-targeting SMARTpool (NT) and SMARTpools for IP3R1 (R1), IP3R2 (R2), and IP3R3 (R3) was insignificant (p > 0.5). C, apoptotic sensitivity to dexamethasone was measured in cells transfected with SMARTpools for each subtype individually. Cells were treated with 100 nm dexamethasone or ethanol vehicle for 24 or 48 h, and apoptosis was measured by flow cytometric quantification of sub-G1 DNA accumulation. Error bars represent the mean ± S.E. of three experiments. In each comparison the dexamethasone-mediated increase in apoptosis was significant (p < 0.01), but in each case knocking down an individual IP3R subtype failed to inhibit apoptosis (p > 0.5).