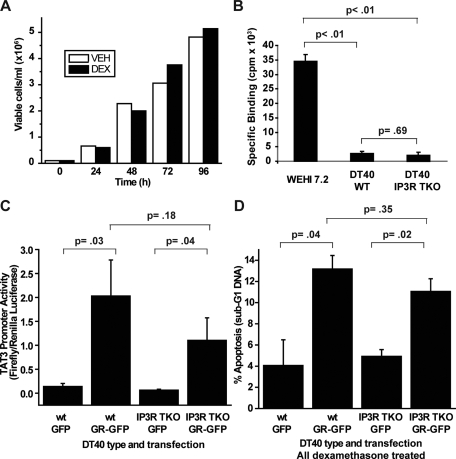
FIGURE 7.
DT40 cells lacking all three IP3R isoforms are not more resistant to dexamethasone-induced apoptosis than wild-type DT40 cells. A, wild-type DT40 cells were treated with (black bars) or without (white bars)1 μm dexamethasone (DEX), and cells were counted 24, 48, 72, and 96 h after treatment. The percentage of viable cells remained ∼100% in both treated and untreated cells at each time point. This experiment is representative of seven independent experiments. VEH, ethanol vehicle. B, a [3H]dexamethasone binding assay was performed on WEHI7.2 cells, wild-type DT40 (WT) cells, and DT40 cells that lacked all three IP3R subtypes (IP3R TKO). Specific binding is expressed in cpm. Error bars represent the mean ± S.E. (n = 3). C, DT40 wild-type cells and IP3R TKO cells were transfected with GFP or GR-GFP, as indicated, as well as the pTAT3-Luc firefly luciferase reporter plasmid and the phRG-TK Renilla luciferase control plasmid. 24 h following transfection, cells were treated with 1μm dexamethasone for 18 h, and Dual-Luciferase reporter assays were performed on the cells. TAT3 promoter activity is expressed as firefly luciferase luminescence divided by Renilla luciferase luminescence. Error bars represent the mean ± S.E. (n = 4). D, DT40 wild-type and IP3R TKO cells were transfected with GFP or GR-GFP, as indicated, and cells were treated with 1 μm dexamethasone. Apoptosis was assessed at 72 h by quantifying sub-G1 DNA accumulation by live cell flow cytometry and gating on GFP-positive cells. Error bars represent the mean ± S.E. (n = 3).