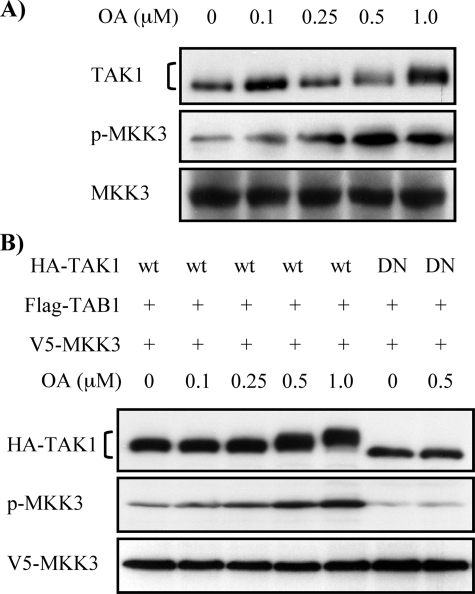
FIGURE 2.
OA-induced TAK1 phosphorylation mediates MKK3 phosphorylation. A, OA treatment increases endogenous MKK3 phosphorylation. MMC grown to subconfluence were treated with increasing concentrations of OA for 6 h as indicated. Cell lysates were subjected to Western blot analysis with anti-TAK1, anti-p-MKK3/6, and anti-MKK3 antibodies, respectively. B, OA-induced MKK3 phosphorylation is dependent on TAK1 activation. Wild-type HA-TAK1 (wt) or kinase-deficient mutant of HA-TAK1 (DN) was coexpressed with FLAG-TAB1 and V5-MKK3 in MMC and treated with increasing concentrations of OA for 6 h. Cell lysates were subjected to Western blot analysis with anti-HA, anti-p-MKK3/6, and anti-V5 antibodies, respectively.