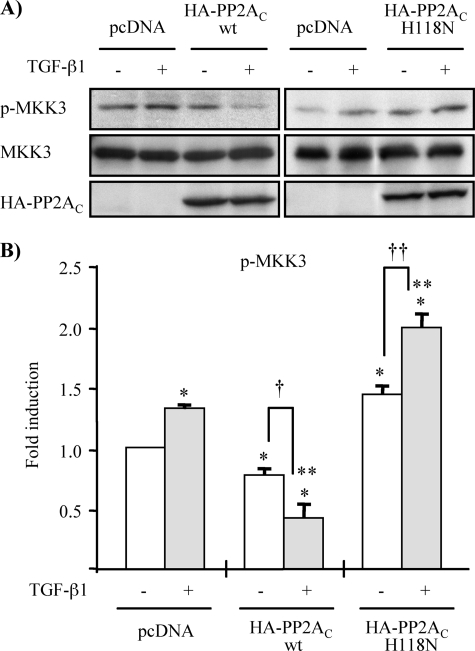
FIGURE 8.
Ectopic expression of wild-type and phosphatase-deficient mutant of PP2AC affects TGF-β1-induced MKK3 phosphorylation. A, MMC were transiently transfected with expression vector encoding wild-type HA-PP2AC (wt) or phosphatase-deficient mutant of HA-PP2AC (H118N), as indicated. As controls, MMC were transfected with empty vector pcDNA3.1 (lanes 1 and 2 of each panel). Following transfection, cells were incubated in medium containing 15% FBS for 18 h and then rendered quiescent in medium supplemented with 0.5% FBS for 16 h, prior to treatment with TGF-β1 (2 ng/ml) for 5 min. Endogenous TAK1 activation was assessed by alteration in MKK3 phosphorylation determined by Western blot analysis with anti-p-MKK3/6 antibody. Immunoblotting with anti-MKK3 and anti-HA antibodies were performed for loading controls. B, densitometry data are presented as -fold increase in p-MKK3, quantified as the ratio to total MKK3, compared with empty vector pcDNA3.1 controls without TGF-β1 treatment; empty vector pcDNA3.1 controls without TGF-β1 treatment (*, p < 0.05 versus empty vector controls without TGF-β1 treatment; **, p < 0.05 versus empty vector controls with TGF-β1 treatment; †, p < 0.05 versus wild-type HA-PP2Ac-transfected cells without TGF-β1 treatment; ††, p < 0.05 versus mutant HA-PP2Ac (H118N)-transfected cells without TGF-β1 treatment).