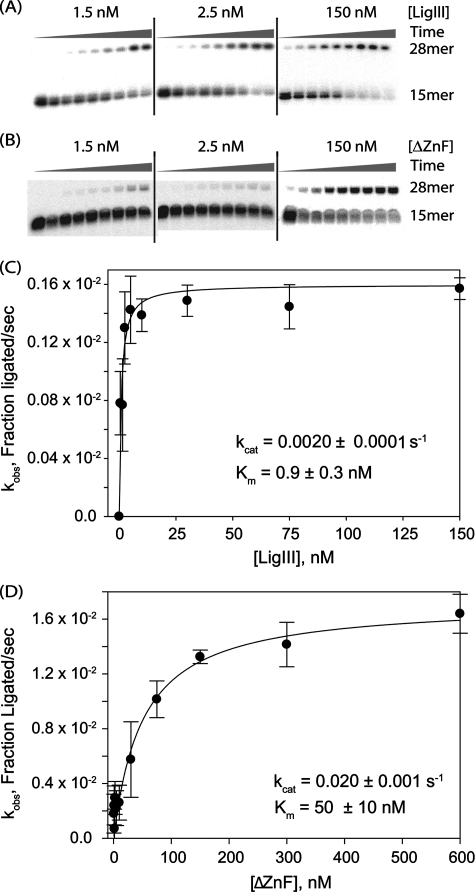
FIGURE 3.
Deletion of the ZnF domain lowers the catalytic efficiency of end joining. A and B, purified LigIII (A) or ΔZnF (B) was assayed for DNA end joining activity in a single turnover assay. The rate of ligation of nicked DNA (0.25 nm) was measured at different enzyme concentrations (1.5, 2.5, 5, 10, 30, 75, 150, 300, or 600 nm). The extent of ligation over time was monitored by periodically removing and quenching aliquots of the reaction (15 s, 30 s, 1 min, 2 min, 4 min, 10 min, 1 h, 2 h, or 3 h) for reactions performed at 0 °C to slow ligation to a measurable rate. C and D, observed rate constants (kobs) for each protein concentration were plotted to determine the rate constant (kcat) and Michaelis constant (Km) for LigIII (C) or ΔZnF (D).