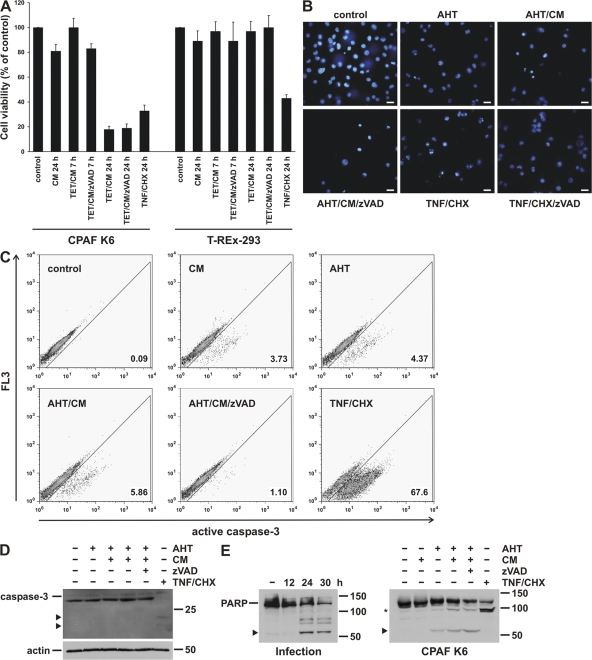
Figure 6.
CPAF expression causes nonapoptotic cell death. (A) CPAF reduces cell viability. CPAF K6 or T-REx-293 cells were treated with the indicated combinations of TET, CM, and the caspase inhibitor zVAD-fmk (zVAD). As a positive control, cells were treated with TNF-α (TNF) and cycloheximide (CHX). After indicated time points, cell viabilities were measured by MTT assay. Relative cell viability was calculated (untreated cells were set to 100%). Data are normalized means/SEM of three independent experiments. (B) Analysis of nuclear morphology after CPAF expression by Hoechst staining. CPAF K6 cells were treated with 10 ng/ml AHT, CM, or zVAD-fmk as indicated. As a positive control of apoptosis, cells were treated with TNF/CHX (as described in A). After 16 h, cells were stained with the Hoechst 33342 dye and analyzed by fluorescence microscopy. Bar, 15 μm. (C) Caspase-3 activation during CPAF-expression. CPAF K6 cells were treated as described in B and analyzed by flow cytometry using an antibody specific for active caspase-3. (D) Analysis of caspase-3 activation by Western blotting. CPAF K6 cells were treated as described in B, and cell extracts were analyzed by Western blotting using an antibody specific for active caspase-3. Arrowheads indicate specific cleavage products of activated caspase-3. Detection of actin served as loading control. (E) Cleavage of PARP by infection with C. trachomatis or expression of active CPAF. (Left) CPAF K6 cells were infected with C. trachomatis for the indicated periods of time. (Right) CPAF K6 cells were treated as described in B. Cell extracts were analyzed by Western blotting using a PARP antibody. The arrowheads indicate cleavage products resulting from chlamydial infection or CPAF expression. The asterisk indicates a specific PARP cleavage product due to caspase activation by TNF/CHX.