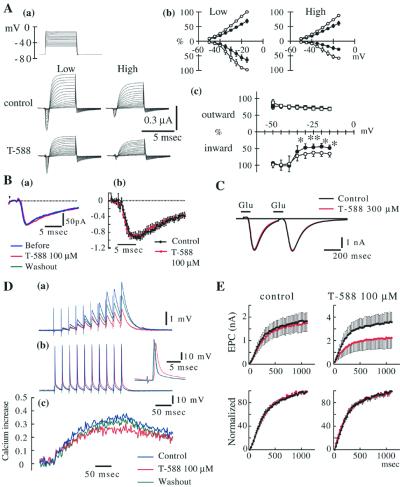
Figure 2.
T-588 in crayfish neuromuscular junction. (A) Axonic voltage-gated currents. (a) Data acquired at low (<1 Hz) and high (40 Hz) voltage step frequency. (b) I–V relationship of control (○) and T-588 (●) (100 μM). (c) Current reduction by T-588. Inward (circles) and outward current (squares) are shown separately. Data represent the mean ± SE, n = 7 experiments. T-588 significantly reduced the inward current at high frequency (closed symbols) and less at low frequency (open symbols) indicating an inward current use-dependent block. *, P < 0.05; **, P < 0.01 probability by paired t test. (B) Single EPC. (a) Single EPC waveforms (recorded before, during T-588, and after washout) are superimposed. (b) Single EPC amplitudes measured every 0.5 msec and normalized (1.0 as peak amplitude in each experiment). Each point in the graph shows mean value of 15 preparations with SE. Black and red lines represent before and during 100 μM T-588, respectively. No change was observed. (C) Postsynaptic glutamate current. Glutamate current generated by paired pulses (at 400-msec intervals) iontophoresis of l-glutamate to a muscle fiber voltage-clamped at the neuromuscular junction. Average wave forms of 10 successive traces during T-588 (300 μM) and after washout are superimposed. (D) Synaptic facilitation and Ca2+ influx. Example waveform of EPPs (a) and presynaptic spikes (b) evoked by 10 repetitive stimulation at 40 Hz and the simultaneously recorded time course of Ca2+ influx (c) at a particular terminal are shown. Calcium uptake was determined from Fura-2 images acquired with two-photon microscopy as an averaged waveform of successive eight measurements. (E) EPC facilitation evoked by 40-Hz repetitive stimulation. (Upper) Absolute amplitude of EPC through 50th stimulus. (Lower) Amplitude is shown as percent to the maximum response in each group. Black and red before and during T-588, respectively. Each point is shown as a mean value with SE, n = 9 experiments.