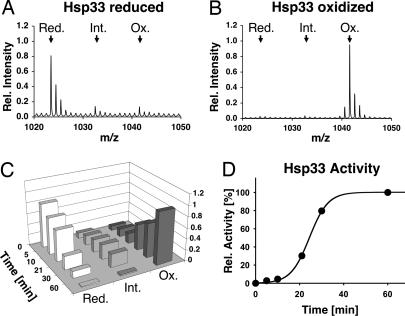
Fig. 2.
Using OxICAT to visualize disulfide bond formation in vitro. Reduced, zinc-reconstituted Hsp33 (50 μM) is incubated in the presence of 2 mM H2O2 at 43°C. Samples are taken at specific time points during the oxidation process, and either TCA precipitated to stop all thiol-disulfide exchange reactions and subjected to the OxICAT method (A–C), or analyzed for chaperone activity (D). (A) Detail of the mass spectrum of OxICAT-treated reduced Hsp33. The two cysteines in peptide 232–236 are almost exclusively labeled with light ICAT (calculated m/z = 1023.4710). (B) Detail of the mass spectrum of OxICAT labeled Hsp33 after 60 min of activation. The two cysteines in peptide 232–236 are mostly labeled with heavy ICAT, leading to a mass shift of 18 Da (calculated m/z = 1041.5314). (C) The monoisotope peak corresponding to the Hsp33 peptide 232–236 in the reduced (red) (m/z = 1023.46), partially oxidized (int) (m/z = 1032.51), or fully oxidized (ox) (m/z = 1041.55) form was plotted against incubation time. (D) Influence of Hsp33 (0.3 μM) on the aggregation of chemically denatured citrate synthase (75 nM) at 30°C. The light scattering signal 4 min after addition of citrate synthase was plotted against incubation time. The light scattering signal of citrate synthase in the absence of Hsp33 was used as 0% chaperone activity, whereas the signal in the presence of fully active Hsp33 was set to 100%.