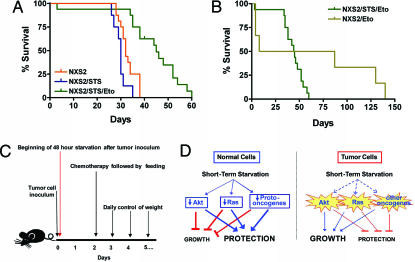
Fig. 5.
DSR in mice. (A and B) Survival of neuroblastoma (NXS2)-bearing mice. All mice were inoculated (i.v.) with 200,000 NXS2 cells per mouse. The different groups were treated as follows: NXS2 (control group, 16 mice), i.v. inoculation with NSX2 tumor cells on time 0; NXS2/STS (STS, 8 mice), i.v. inoculation with NSX2 tumor cells at time 0 followed by a 48-h starvation; NXS2/STS/Eto (STS/Eto, 16 mice), i.v. inoculation with NSX2 tumor cells at time 0, followed by a 48-h starvation, followed by an i.v. injection with 80 mg/kg etoposide and feeding at 48 h; NXS2/Eto (Eto, 6 mice, two deaths caused by the injection procedure), i.v. inoculation with NSX2 tumor cells at time 0, followed by an i.v. injection of 80 mg/kg etoposide at 48 h. The survival period of the NXS2 (control) and NXS2/STS/Eto groups was significantly different (P < 0.001), whereas that of the NXS2 (control) and Eto groups was not (P = 0.20). In addition, the survival periods of the NXS2/STS/Eto and NXS2/Eto groups were not significantly different (P = 0.12). (C) Procedure for the in vivo experiment. (D) Model for DSR in response to STS. In normal cells, downstream elements of the IGF1 and other growth factor pathways, including the Akt, Ras, and other proto-oncogenes, are down-regulated in response to the reduction in growth factors caused by starvation. This down-regulation blocks/reduces growth and promotes protection to chemotherapy. By contrast, oncogenic mutations render tumor cells less responsive to STS because of their independence from growth signals. Therefore, cancer cells fail to or only partially respond to starvation conditions and continue to promote growth instead of protection against oxidative stress and high-dose chemotherapy.