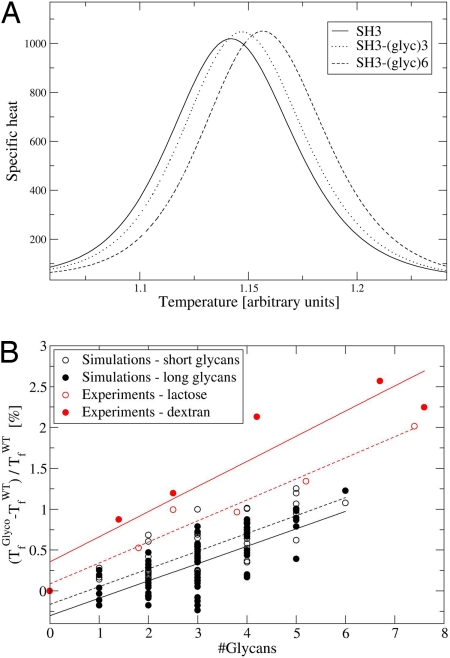
Fig. 3.
Thermal stabilization of the glycosylated variants of the SH3 domain. (A) Specific heat curves of native SH3 (filled line) and of SH3 modified with three (dotted line) and six (dashed line) 11-sugar ring glycans. (B) Effect of glycosylation on protein stability as measured by the change in the Tf. Simulations of 63 SH3 glycosylated variants bearing short glycans comprised 5 sugar rings (open black circles, fitted by a dashed black line; R = 0.805) or long glycans comprised of 11 sugar rings (filled black circles, fitted by a solid black line; R = 0.719). Experimental measurements of stabilization by chemical glycosylation of α-CT (27) with lactose (open red circles, fitted by a dashed red line; R = 0.98) or 10-kDa dextran (filled red circles, fitted by a solid red line; R = 0.936) from the work of Sola et al. (27, 28) are shown. The number of glycans conjugated to α-CT was calculated as the average moles of glycan per mole of α-CT.