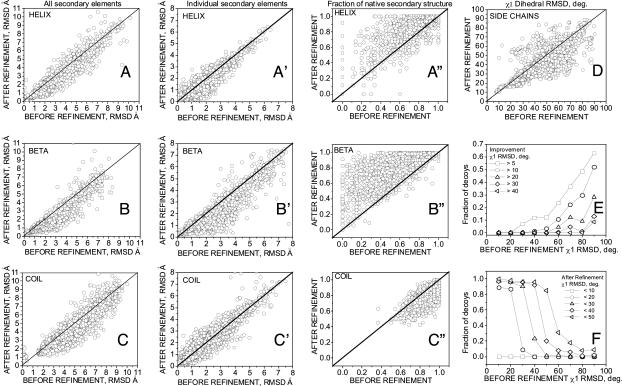
Fig. 2.
Results of decoy structure refinement within each trajectory (100 decoys per protein, 39 proteins) for different structural elements. (A–C and A′–C′) Cα rmsd to the native structure of the lowest-energy decoy after refinement versus the decoy's initial Cα rmsd, for helical (A), β-sheet (B), and loop (C) regions when all the native secondary structure elements of a given type are superimposed together onto the corresponding decoy regions, and for helices (A′), β-sheets (B′), and loops (C′) when individual secondary structure elements from the native are superimposed onto related decoy regions. (A″–C″) Comparison of fractions of the native secondary structure in the refined (lowest-energy decoy from each trajectory) and the initial decoy, for helical (A″), β-sheet (B″), and loop (C″) regions. (D–F) Refinement of packing of buried side chains. (D) χ1 rmsd (degrees) to the native structure of the lowest-energy decoy after refinement with respect to χ1 rmsd of the initial structure. (E and F) Fraction of the lowest-energy decoys that improved their χ1 rmsd by more than (E) or below (F) a given threshold with respect to χ1 rmsd of the initial structure. Fractions of decoys were calculated in 10° bins.