Abstract
We used a phylogenetically based comparative approach to evaluate the potential for physiological studies to reveal patterns of diversity in traits related to susceptibility to an environmental stressor, the trace metal cadmium (Cd). Physiological traits related to Cd bioaccumulation, compartmentalization, and ultimately susceptibility were measured in 21 aquatic insect species representing the orders Ephemeroptera, Plecoptera, and Trichoptera. We mapped these experimentally derived physiological traits onto a phylogeny and quantified the tendency for related species to be similar (phylogenetic signal). All traits related to Cd bioaccumulation and susceptibility exhibited statistically significant phylogenetic signal, although the signal strength varied among traits. Conventional and phylogenetically based regression models were compared, revealing great variability within orders but consistent, strong differences among insect families. Uptake and elimination rate constants were positively correlated among species, but only when effects of body size and phylogeny were incorporated in the analysis. Together, uptake and elimination rates predicted dramatic Cd bioaccumulation differences among species that agreed with field-based measurements. We discovered a potential tradeoff between the ability to eliminate Cd and the ability to detoxify it across species, particularly mayflies. The best-fit regression models were driven by phylogenetic parameters (especially differences among families) rather than functional traits, suggesting that it may eventually be possible to predict a taxon's physiological performance based on its phylogenetic position, provided adequate physiological information is available for close relatives. There appears to be great potential for evolutionary physiological approaches to augment our understanding of insect responses to environmental stressors in nature.
Keywords: comparative methods, evolutionary physiology, bioaccumulation, phylogeny, tradeoff
With ≈6,500 species described to date in North America (1), aquatic insects are a diverse and ecologically important group (2), particularly in rivers and streams. For example, the orders Ephemeroptera, Plecoptera, and Trichoptera (EPT taxa) include 58 recognized families and ≈2,700 species (1). Among these many lineages, great diversity exists in morphology, life history characteristics, and physiology stemming from a long and complex evolutionary history. Although the origins of the Ephemeroptera are unknown (3), a general paradigm of the terrestrial ancestry of aquatic insects is widely accepted, with numerous invasions of freshwater habitats hypothesized throughout evolutionary history (4). Many of these invasions have entailed adaptive “solutions” that involve complex suites of traits that in combination determine the range of environmental conditions that a given taxon can tolerate.
Some traits that arose in response to past environmental challenges may now render certain species relatively more susceptible to modern anthropogenic pollutants. These pollutants may be either entirely novel (e.g., organophosphate insecticides) (see ref. 5) or were historically present at much lower concentrations in natural environments than they are in many ecosystems today (e.g., trace metals) (6). This variation in susceptibility has practical implications, because the ecological structure of aquatic insect communities is often used to indicate the ecological conditions in freshwater systems (7–9). Differences among species' responses to environmental stressors can be profound, but it is uncertain whether the cause is related to functional ecology [usually the assumption (10, 11)] or physiological traits (5, 12–14), which have received considerably less attention. To the degree that either is involved, their link to phylogeny and evolutionary history remains poorly understood.
Here, we ask whether the tendency for related species to be similar (i.e., phylogenetic signal) (15, 16) extends to physiological traits that contribute to sensitivity to the stressor: cadmium. Generalizations about phylogenetic linkages to stress responses have been hindered to date by the limited number of species that can be studied. We overcome this hurdle by using highly developed methodologies for efficiently quantifying critical processes that control sensitivity to dissolved cadmium (see refs. 13, 17, and 18) (Fig. 1). These physiological processes have previously been considered in a conceptual model of metal toxicity (19) that explicitly assumes that chronic toxicity in nature is the manifestation of metal accumulation at target sites (i.e., metal-sensitive sites) that ensues when the rate of metal influx exceeds the combined rates of metal excretion and detoxification. In practice, the model can be tested by combining bioaccumulation kinetics (see ref. 17) and subcellular fractionation (e.g., refs. 18 and 20). We have used this integrated approach to reconcile apparent discrepancies between insect responses to trace metals in toxicity assays and in nature (13), to infer Cd sensitivity differences among predaceous stoneflies (14), and to understand the mechanisms underlying the metal tolerance of a caddis fly (21).
Fig. 1.
Conceptual model of metal toxicity, adapted in part from ref. 19. Here we consider uptake (solution), elimination, and detoxification in the prediction of dissolved Cd susceptibility among species.
We used phylogenetic analyses (22–30) to explore physiological processes related to dissolved Cd susceptibility in 21 field-collected aquatic insect species representing eight EPT families [supporting information (SI) Table S1]. We tested for correlations and possible trade-offs among traits and used traits in combination to predict the more emergent property (sensitivity) in each of these species. We asked whether phylogenetic approaches (15, 16, 22–30) are potentially more powerful than traditional functional guild approaches (e.g., ref. 31) for predicting sensitivity differences among species. We compared statistical models that include body weight, feeding strategy, and lineage as independent variables by using both conventional and phylogenetically based statistical tools. Finally, we determined how phylogenetically based approaches can increase our understanding and prediction of interspecific susceptibility differences and augment the interpretive power of conventional stream assessments that use insects as ecological indicators.
Results
Cd Uptake from Solution (ku).
Under identical water chemistry conditions, cadmium uptake rate constants (ku) varied 65-fold among the 21 species we studied (Fig. 2A). We used Blomberg et al.'s (15) K statistic to quantify the tendency for related species to be similar to each other (phylogenetic signal) in ku measurements. In general, the level of phylogenetic signal present in our Cd uptake rate constants (K = 0.488; P = 0.031) was typical for physiological traits quantified in other studies. Blomberg et al. report that the average K value taken from 21 comparative physiology data sets was 0.53 (95% confidence interval 0.40–0.72). Because many physiological traits are expected to scale allometrically, we also determined K after removing the influence of log body weight (K = 0.482; P = 0.041).
Fig. 2.
Cadmium uptake rate constants (ku ± SE) (A) and elimination rate constants (ke ± SE) (B) derived from radiotracer experiments with 21 aquatic insect taxa representing the orders Ephemeroptera, Plecoptera, and Trichoptera. Pagel's arbitrary branch lengths (depicted) were used as input phylogenies in statistical analyses.
To explore the role of phylogeny in explaining Cd uptake rate differences among species, we compared 18 regression models that included combinations of body weight, clade (families or orders), and feeding type (Table S2). The best-fit regression model for log ku measurements based on the Akaike Information Criterion (AIC) included log body weight (not significant) and family categories (P = 0.012) on a star phylogeny, thus demonstrating a significant phylogenetic component to dissolved Cd uptake. (Note: A star phylogeny assumes all taxa are equally related as in traditional statistical methods, whereas a tree includes hierarchical structure.) This pattern indicates that the phylogenetic component of variation for uptake is associated primarily with differences among families, the lowest phylogenetic level for which we have adequate sample size and meaningful hierarchical structure in our dataset. Body weight [which also exhibited a statistically significant phylogenetic signal for log values (K = 0.532; P = 0.011)] was negatively correlated with log ku, but the statistical significance of family was not affected by the inclusion of log weight in regression models. The superiority of models including family as a factor over models including orders demonstrates that, within orders, there is considerable variation in uptake rate constants, which is apparent when the raw data are plotted onto the phylogeny of our tested species (Fig. 2).
Within orders, ku values ranged 20-fold among 7 mayfly taxa, 25-fold among 10 stonefly taxa, and 38-fold among 4 caddis fly taxa. Among mayflies, ephemerellids had consistently faster Cd uptake than both isonychiids and heptageniids, and species in any given family tended to be more similar to their family members than they were to species in other families. Among stoneflies, perlodids had uniformly slow Cd uptake relative to perlids, with the latter group exhibiting a 7-fold range in ku values. Among caddis flies, Rhyacophila congeners were similarly slow in their Cd uptake. Interestingly, the two hydropsychids we examined were radically different with uptake in Hydropsyche californica (0.42 liter·g−1·d−1) >10-fold faster than in Cheumatopsyche sp. (0.04 liter·g−1·d−1). This pattern serves as a counterpoint to the general pattern for related species to be somewhat similar. Further examination of physiological variability is clearly warranted in this large family. We also explored feeding in relation to Cd uptake, but this trait was not significant in any ku models (Table S2).
Cadmium Elimination (ke).
In general, uptake rate constants alone were not necessarily strong predictors of bioaccumulation, because rates of elimination are very important in this process. Elimination rate constants (ke) are perhaps the most important determinant of its overall bioaccumulation in nature, because they apply to both dissolved and dietary accumulation (17). The ke values we present here represent a daily proportional loss of Cd from the body during the slow phase of efflux, which is thought to be more representative of loss rates under chronic exposure conditions found in nature (32). Efflux rate constants ranged ≈25-fold among species in which we could measure loss (Fig. 2B). Log ke estimates exhibited a much stronger phylogenetic signal (K = 0.595; P = 0.013) than did log ku. This difference was accentuated by removing the influence of log body weight (K = 0.753; P < 0.001). Thus, relative to other physiological traits (15), Cd ke values exhibit a very strong phylogenetic signal. Furthermore, ke is strongly influenced by body size.
To explore the role of phylogeny in explaining Cd uptake rate differences among species, we compared regression models as described above (Table S3). The best-fit regression model based on AIC included log (body weight) (P < 0.01) and family categories (P < 0.01), demonstrating a significant phylogenetic component to Cd elimination. One model containing only feeding and weight in the absence of any phylogenetic information showed feeding to be statistically significant. However, feeding was not significant when family categories were included in the model, perhaps because feeding type tended to be uniform within families.
In our studies, taxa exhibiting the extremes of ke estimates are instructive, because they represent some of the few groups for which generalizations of metal susceptibility in the field are available. For example, both heptageniid mayflies we examined did not exhibit any significant loss of Cd from their tissues over 10 days of efflux (Fig. 2B). Heptageniids have been described as particularly sensitive to trace metal exposures in the field, and are often extirpated from contaminated systems (33, 34). On the other hand, H. californica exhibited one of the highest efflux rates measured yet in any invertebrate (0.20) (21), and this genus is often described as being metal tolerant (33). Interestingly, the ke value for H. californica is very close to that measured by Evans et al. (35) in Hydropsyche bettini, yet we also see “phylogenetic antisignal” (in the sense of ref. 15) when we compare H. californica to Cheumatopsyche sp., despite the fact that they are in the same family (Fig. 2B).
Subcellular Cd Compartmentalization.
Because bioaccumulation alone is not necessarily a good predictor of sensitivity differences among species (Fig. 1; refs. 18, 19), it is important that bioaccumulation models are linked to other approaches to better understand the potential biological consequences of accumulated metals in a given species. The subcellular compartmentalization of accumulated metals gives some insight into the potential for toxicity (18), because species vary in their abilities to protect cells with metal-binding proteins such as metallothionein-like proteins (13), glutathione, and metal-rich granules (33). In our studies, the subcellular compartmentalization of Cd varied markedly among taxa (Table S4). We were particularly interested in Cd associated with microsomes, organelles, and heat-labile cytosolic proteins, because those compartments represent greater potential for Cd to interfere with vital physiological processes. Cd associated with the heat-stable protein (HSP) compartment has previously been assumed to be detoxified via metallothionein-like proteins (36, 37) and glutathione. Cd associated with cell debris was not considered to have toxicological significance to the larvae.
On average, there were no major differences among orders in terms of the proportion of Cd accumulated in potentially sensitive compartments (microsomes, organelles, and heat-labile cytosolic proteins). For all taxa tested, the average percentage (± SD) of accumulated Cd in these compartments was 36 ± 14%. The perlid stonefly Paragnetina sp. had the smallest percentage (12%) of its body burden in these compartments, whereas the perlodid stonefly Isogenoides hansonii had the largest (64%). There was considerable variation within orders, families, and among genera, and no apparent trends in Cd compartmentalization into sensitive compartments was observed. Although there was an apparent difference in the proportion of Cd associated with the HSP compartment among orders (conventional one-way ANOVA: F = 4.21; df = 2, 18; P = 0.032) and families (F = 3.03; df = 7, 13; P = 0.040), these differences were not even close to significant in the equivalent phylogenetic analyses [phylogenetic generalized least-squares (PGLS): F = 0.15, df = 2, 18, P = 0.860; F = 0.37, df = 7, 13, P = 0.901, respectively]. Of possible biological significance is the observation that mayflies tended to accumulate higher percentages (≈39%) of Cd in this compartment than did stoneflies (≈18%) and caddis flies (17%). Phylogenetic signal in this trait (K = 0.475; P = 0.057) was typical for physiological traits (see above).
Correlations/Linkages Among Traits.
The nonphylogenetic (star) correlation between cadmium uptake (log ku) and elimination (log ke) was 0.141 [ln maximum likelihood (ML) = −16.2238; P = 0.5435], and this correlation remained nonsignificant with the effects of log body weight controlled (ln ML for model = −14.1105; partial r = 0.225; P = 0.3395). However, the phylogenetic correlation (tree) between log ku and log ke was larger, 0.357 (ln ML = −13.6871, P = 0.1120) and became statistically significant when the effects of log body weight were controlled (ln ML for model = −8.05635; partial r = 0.505; P = 0.0232). Comparison of the likelihoods indicates that the phylogenetic models better fit the data, and that the phylogenetic model including log body weight is the best, significantly better than the version without log body weight (ln likelihood ratio test, χ2 with 1 df = 11.26; P = 0.0008). Therefore, size-independent log ku and log ke appear to have evolved together in a correlated fashion.
Among all species, elimination rate constants (ke) and the proportion of Cd bound to the HSP (detoxified) compartment were negatively correlated regardless of whether ke was log-transformed or phylogeny was considered. The best models based on AIC used a star phylogeny for log ke (r = −0.80; P < 0.0001). This relationship was driven by the strong tendency within the Ephemeroptera (r = −0.89; P < 0.01), suggesting that mayfly taxa that do not efflux Cd effectively, instead allocate considerable resources toward detoxifying Cd (Fig. 3). No other relationships between biokinetic parameters and the proportional intracellular distribution of Cd were found.
Fig. 3.
Inverse relationship between the ability to eliminate Cd from tissues (log ke) and the association of Cd with HSPs. ●, Ephemeroptera; ■, Plecoptera; ▴, Trichoptera.
Estimating Susceptibility Differences.
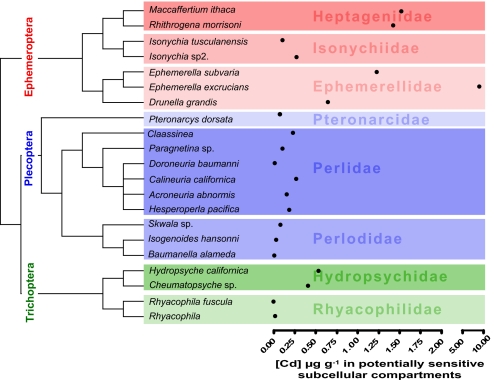
As described (13), we predicted interspecific differences in dissolved Cd sensitivity by integrating kinetic parameters (annual Cd bioconcentration estimates) with estimates of detoxification capacity (the proportion of accumulated Cd that was measured in potentially sensitive subcellular fractions) (Fig. 4). This approach is aligned with a critical residue approach for regulating trace metal inputs to surface waters. These values for predicted metal concentrations at potentially sensitive sites ranged over three orders of magnitude among all taxa. This integrated estimate had a strong phylogenetic signal (K = 0.718; P < 0.01), which was even stronger after removing the influence of body weight (K = 0.767; P < 0.01).
Fig. 4.
Identification of Cd-susceptible taxa. Uptake and elimination rate constants were used to model Cd accumulation from exposure to dissolved Cd (0.50 μg/liter) for 1 year. The bioaccumulation estimates were modified to only consider the proportion of accumulated Cd associated with microsomes, organelles, and heat-labile cytosolic proteins measured in subcellular compartmentalization studies.
To explore the role of phylogeny in explaining susceptibility differences among species to dissolved Cd, we compared regression models as described above (Table S5). The best-fit regression model based on AIC included weight (P = 0.017) and family (P < 0.001), indicating a strong phylogenetic component to Cd susceptibility. In addition, it appears that within a given family, smaller-bodied species tend to be more sensitive to Cd than larger ones.
Discussion
Our primary purpose was to evaluate the potential for comparative studies to reveal patterns of diversity in traits related to susceptibility to a stressor. Our approach differs from traditional trait-based work (10, 11, 38) in insects in two major ways. First, we focus on physiological traits rather than ecological traits. We show that variability among taxa in susceptibility to a given level of stress, as defined by bioaccumulation into potentially toxic sites, can be strongly driven by phylogenetically linked physiological traits. Functional traits such as choice of food or seasonal aspects of development could result in different exposures in the same habitat or the presence of sensitive life stages during periods of elevated metal exposure (spring run-off for example). However, here we show that the mechanistic linkage between physiology and a taxon's performance is powerful in the widely divergent aquatic insects we studied. Second, we adopt a phylogenetic perspective rather than a functional guild approach, because the tendency for related organisms to be similar to one another is pervasive (15, 16). Although we were specifically interested in testing whether this phylogenetic signal holds true for physiological traits related to Cd susceptibility, the approach may be applicable to susceptibility to other stressors as well.
Individual traits related to Cd bioaccumulation exhibited phylogenetic signal (elimination more so than uptake). This tendency for related animals to be physiologically similar has practical implications. If widespread, it may be used to overcome the inherent limitation in the number of species that can be studied in physiological experiments. It may eventually be possible to predict physiological performance in a taxon that has yet to be studied on the basis of its phylogenetic position, provided sufficient information is available for close relatives (e.g., see refs. 15 and 26). The current dataset is probably insufficient for making predictions beyond the family level (because of limited sample size and hierarchical structure within families). However, it is tempting to think that relatively fast uptake in ephemerellid mayflies or poor elimination in heptageniid mayflies could be hallmarks of those families. It is possible that more phylogenetically focused comparative studies, within a given family, for example, might allow for reasonable predictions for all members of that family (see ref. 26).
In combination, traits related to bioaccumulation and detoxification appeared to identify metal-sensitive species. Our studies identified the genus Ephemerella and two heptanegiid mayflies as being particularly susceptible to dissolved Cd. These findings give mechanistic support to observations that these taxa are consistently absent from metal-contaminated habitats in nature (40) and are consistently more sensitive to metals in sophisticated toxicity tests (39–41). The presence of a relatively strong phylogenetic signal associated with the integrated trait of “sensitivity to dissolved Cd” suggests that sensitivity to metals may eventually be predictable on the basis of phylogenetic position, as described above. Given that this design was not a “common garden” experimental design (42), and species came from various locations in the United States, the degree of phylogenetic signal associated with these data is encouraging.
We found a positive correlation between the bioaccumulation parameters ku and ke, but only after controlling for the influence of body weight and considering phylogeny. We offer two potential explanations, not mutually exclusive (23), for why these two traits may have evolved in a correlated fashion. First, they may be positively genetically correlated, perhaps because some genes pleiotropically affect transporter densities in parallel in some tissues or organs. Quantitative-genetic analyses could test this hypothesis. Second, selection may have favored their correlated evolution. Studies of selection in laboratory mesocosms could test this idea. We also found a potential tradeoff between the ability to eliminate Cd and the ability to detoxify it and speculate that in insects an enhanced ability to eliminate may be a better strategy than expressing proteins or peptides for detoxification. The storage of metals in extracellular granules could be an efficient means of evading toxicity (43, 44), but we were unable to differentiate Cd associated with chitin with granules in our studies. Species that do well at eliminating, detoxifying, and/or storing metals, such as H. californica, are able to thrive in metal-rich environments (21).
The most reliable estimates of metal susceptibility still come from field-based observational approaches, where metals are usually present in mixtures, and dietary pathways of accumulation may in fact predominate in predatory insects (e.g., refs. 14, 45, and 46). In light of these complexities, it is somewhat surprising that our predictions of susceptibility to dissolved Cd so closely match field-based observations. We offer a few possible explanations for this. First, the efflux rate constant ke applies to both dissolved and dietary exposure routes (14) and is arguably the most important determinant of bioaccumulation (17). Second, we have already established that uptake rates of Cd and zinc (Zn) covary strongly among species (12), so our rate constants of Cd uptake are likely also reasonable approximations for Zn. Third, recent work on mayflies (Family Heptageniidae and Ephemerellidae) using stable Cu and Cd isotopes as tracers suggest that transport rates of these elements covary (D.J.C., unpublished work). It will obviously be important to better understand the contributions of dietary metal bioaccumulation and improve on our ability to assess coping mechanisms and toxicity (Fig. 1).
Knowing which taxa are likely to be susceptible to specific anthropogenic disturbances would greatly enhance the interpretive power of biomonitoring and bioassessment programs, because it may allow species presence/absence or density to diagnose the causes of ecological impairment in aquatic systems. For many common environmental stressors, particularly those that directly affect insect physiological processes, such understanding appears possible through comparative physiological approaches (30, 47). Developing predictive capabilities based on the underlying physiological processes that determine species' responses to water quality would nicely complement other strategies that have traditionally focused on population and community-level responses.
Materials and Methods
Insect Collecting and Handling.
All of the insect larvae used in these studies were field-collected from streams in Northern California, Colorado, Oregon, and North Carolina by using a D-frame kick net (Table S1). Species were selected based on phylogenetic considerations and local availability. Sites were chosen to minimize the potentially confounding influence of metal exposure history (21) (high-quality streams supporting a diversity of insects). Insects were held in an environmentally controlled room with a light/dark photoperiod of 16:8 h and a constant temperature of 15°C. Soft artificial river water (48 mg/liter NaHCO3, 30 mg/liter CaSO4·2H2O, 30 mg/liter MgSO4, and 2 mg/liter KCl, adjusted to pH 6.85 by additions of 0.1 N NaOH) was used for acclimation (at least 4 days) to laboratory conditions and experiments. During acclimation, insects were fed a diet consisting of alfalfa, Spirulina aquarium flake food (O.S.I. Marine Lab), TetraMin fish food, or Lumbriculus variegatus ad libitum depending on the dietary requirements of each taxon. Insects were not fed for 1 day before experiments to reduce fecal material output and metal sorption onto fecal material during aqueous exposures. Only apparently healthy, intact, active animals were used in these experiments. A phylogeny of test species was compiled based on trees assembled on the Tree of Life web site (www.tolweb.org/tree). Physiological measures were mapped onto this phylogeny.
Physiological Experiments.
The methods used in these physiological studies have been reported (13, 21). Larvae were exposed to an environmentally relevant concentration of Cd (≈4.6 nM) in artificial soft water. The gamma-emitting isotope 109Cd was used to follow Cd accumulation and efflux kinetics in vivo by using a PerkinElmer Wallac Wizard 3-inch gamma counter. The accumulation phase of the experiment lasted 5 days for all taxa except for experiments with Rhyacophila sp. and H. californica (6 days). Approximately half of the larvae were used to conduct elimination experiments lasting 9–12 days. Briefly, insects were maintained in Cd-free water and assayed daily to monitor the loss of radioactivity from each larva. Efflux of Cd generally followed a biphasic pattern, and we focused on the slow phase of efflux as it more likely represents loss during long-term exposure scenarios (32, 48). Therefore, loss constants (ke) were estimated by fitting efflux data after the first day of loss.
Subcellular Fractionation.
After the accumulation phase of the experiment, approximately half of the larvae were assayed for 109Cd and homogenized in refrigerated, N2-saturated, 0.05 M Tris·HCl buffer (pH 7.4). The homogenate was subsequently separated by differential centrifugation and chemical and heat treatments into five operationally defined subcellular fractions following described procedures (20, 21, 33, 49). These fractions included cell debris, cellular organelles, microsomes, cytosolic proteins denatured by heat treatment (heat-denatured proteins, HDPs), and HSPs. In previous papers, we have referred to these cytosolic protein fractions as metallothionein-like proteins and nonmetallothionein-like proteins (13, 14, 21). Here, we have changed our nomenclature because our recent work has failed to demonstrate the presence of cysteine-rich, metallothionein-like proteins in the HSP fractions of various aquatic insects. Rather, it seems that this fraction is dominated by glutathione (L.X. and D.B.B., unpublished work). We maintain the convention of assuming that Cd in the HSP fraction represents detoxified Cd (36, 50). The heat-denatured fraction represents a variety of larger cytosolic proteins (20). Data from the subcellular fractions were summed into operationally defined metal-sensitive and detoxified compartments. The metal-sensitive compartment comprised the HDP, microsomal, and organellar fractions, each containing sites potentially vulnerable to Cd binding. The detoxified metal compartment was Cd associated with HSP. Cd in the cell debris fraction was interpreted as being associated with metal-rich granules or biologically inert tissue such as chiton and was not considered toxicologically important.
Statistics.
For a univariate measure of phylogenetic signal, we computed the K statistic of Blomberg et al. (15) and used their randomization test for statistical significance, based on the mean-squared error (their Matlab PHYSIG.m program). We also applied these procedures after removing correlations with log body weight as described (15).
To elucidate whether phylogenetic signal was pervasive (distributed throughout the phylogenetic tree for the species under study) or occurred mainly in relation to disjunctions among orders or families, we compared regression models that included log body weight and either order or family (both coded as K − 1 dummy variables, where K is the number of orders or families) as independent variables. We included body weight because, on first principles, many physiological traits are expected to scale allometrically. For consistency among traits we retained body weight in models even when it was not statistically significant (i.e., P > 0.05). Alternative models were also computed for either a star phylogeny [no hierarchical structure, as is assumed by conventional statistical analyses (26, 48)] or the specified phylogenetic tree (PGLS), using Pagel's arbitrary branch lengths as implemented in the DOS PDTREE program (27). In addition, for the hierarchical phylogenies, we allowed branch lengths to vary between those indicated by the original input tree and a star phylogeny, in a way consistent with an Ornstein-Uhlenbeck (OU) model of (residual) trait evolution. Finally, we ran models that included feeding strategy as a quantitative trait, scored as 0 = nonpredator, 0.5 = intermediate, 1 = predator (see Table S1). All models were analyzed by using the Regressionv2.m Matlab program of Lavin et al. (30).
As an indicator of the relative support of models, we examined the AIC, using the smaller-is-better formulation [AIC = (−2 × ln ML) + (2 × no. of parameters)]. When comparing a series of models, the one with the lowest AIC is considered to provide the best fit to the data. As a rule of thumb, models whose AIC is ≤2 units larger can also be said to have substantial support (29, 30, 51). Note that MLs are used for computing the AIC, whereas restricted maximum likelihood (REML) is used for estimating coefficients in the model, such as the allometric scaling exponent and the OU transformation parameter, d.
To test for correlations between traits, we computed phylogenetically independent contrasts in the DOS PDTREE program (26, 27). To control for the effects of log body weight, we computed partial correlations (through the origin) with the contrasts using the REGRESSION procedure of SPSS version 11.5. We compared ln MLs (from Regressionv2.m) to determine which models (phylogenetic or not, with or without log body weight) best fit the data.
Supplementary Material
Acknowledgments.
We thank Phil Rainbow, Leah Bêche, and Gerald LeBlanc for commenting on early drafts of this manuscript; anonymous reviewers for improving the clarity of its presentation; Will Clements (Colorado State University, Fort Collins) for C. sabulosa; Dave Penrose (North Carolina State University) and Steve Fend (U.S. Geological Survey) for taxonomic support; and Ashley Dale and Jen Flippin for contributions to graphics. This work was supported by a National Research Council postdoctoral fellowship (to D.B.B.) and start-up funds provided by North Carolina State University (to D.B.B.). T.G. was supported by National Science Foundation Grant DEB-0416085.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801686105/DCSupplemental.
References
- 1.Merritt RW, Cummins KW, Berg MB, editors. An Introduction to the Aquatic Insects of North America. Dubuque, IA: Kendall-Hunt; 2008. [Google Scholar]
- 2.Wallace JB, Webster JR. The role of macroinvertebrates in stream ecosystem function. Annu Rev Ent. 1996;41:115–139. doi: 10.1146/annurev.en.41.010196.000555. [DOI] [PubMed] [Google Scholar]
- 3.Barber-James HM, Gattolliat J-L, Sartori M, Hubbard MD. Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia. 2008;595:339–350. [Google Scholar]
- 4.Kristensen NP. Phylogeny of insect orders. Annu Rev Entomol. 1981;26:135–157. [Google Scholar]
- 5.Buchwalter DB, Jenkins JJ, Curtis LR. Respiratory strategy is a major determinant of [3H]water and [14C]chlorpyrifos uptake in aquatic insects. Can J Fish Aquat Sci. 2002;59:1315–1322. [Google Scholar]
- 6.Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water, and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- 7.Barbour MT, Gerritsen J, Snyder BD, Stribling JB. Rapid Bioassessment Protocols for Use in Wadeable Streams and Rivers: Periphyton, Benthic Macroinvertebrates, and Fish. Washington, DC: Environmental Protection Agency; 1999. EPA Publ No 841-B-99-002. [Google Scholar]
- 8.Rosenberg DM, Resh VH, King RS. Use of aquatic insects in biomonitoring. In: Merritt RW, Cummins KW, Berg MB, editors. An Introduction to the Aquatic Insects of North America. Dubuque, IA: Kendall/Hunt; 2008. pp. 123–138. [Google Scholar]
- 9.Wright JF. An introduction to RIVPACS. In: Wright JF, Sutcliffe DW, Furse MT, editors. Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques. Ableside, UK: Freshwater Biological Association; 2000. pp. 1–24. [Google Scholar]
- 10.Poff NL, et al. Functional trait niches of North American lotic insects: Trait-based ecological applications in light of phylogenetic relationships. J North Am Benthol Soc. 2006;25:730–755. [Google Scholar]
- 11.Statzner B, Hildrew AG, Resh VH. Species traits and environmental constraints: Entomological research and the history of ecological theory. Annu Rev Ent. 2001;46:291–316. doi: 10.1146/annurev.ento.46.1.291. [DOI] [PubMed] [Google Scholar]
- 12.Buchwalter DB, Luoma SN. Differences in dissolved cadmium and zinc uptake among stream insects: Mechanistic explanations. Environ Sci Technol. 2005;39:498–504. doi: 10.1021/es0404421. [DOI] [PubMed] [Google Scholar]
- 13.Buchwalter DB, Cain DJ, Clements WH, Luoma SN. Using biodynamic models to reconcile differences between laboratory toxicity tests and field biomonitoring with aquatic insects. Environ Sci Technol. 2007;41:4821–4828. doi: 10.1021/es070464y. [DOI] [PubMed] [Google Scholar]
- 14.Martin CA, Cain DJ, Luoma SN, Buchwalter DB. Cadmium ecophysiology in seven stonefly (Plecoptera) species: Delineating sources and susceptibility. Environ Sci Technol. 2007;41:7171–7177. doi: 10.1021/es071205b. [DOI] [PubMed] [Google Scholar]
- 15.Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution (Lawrence, Kans) 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 16.Freckleton RP, Harvey PH, Pagel MD. Phylogenetic analysis and comparative data: A test and review of evidence. Am Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 17.Luoma SN, Rainbow PS. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol. 2005;39:1921–1931. doi: 10.1021/es048947e. [DOI] [PubMed] [Google Scholar]
- 18.Wang WX, Rainbow PS. Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ Chem. 2006;3:395–399. [Google Scholar]
- 19.Rainbow PS. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ Pollut. 2002;120:497–507. doi: 10.1016/s0269-7491(02)00238-5. [DOI] [PubMed] [Google Scholar]
- 20.Wallace WG, Lee B-G, Luoma SN. The subcellular compartmentalization of Cd and Zn in two bivalves. I. The significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM) Mar Ecol Prog Ser. 2003;249:183–197. [Google Scholar]
- 21.Cain DJ, Buchwalter DB, Luoma SN. The influence of exposure history on the bioaccumulation and subcellular distribution of aqueous cadmium in the insect Hydropsyche californica. Environ Toxicol Chem. 2006;25:1042–1049. doi: 10.1897/05-255r.1. [DOI] [PubMed] [Google Scholar]
- 22.Avise JC. Evolutionary Pathways in Nature: A Phylogenetic Approach. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 23.Garland T, Jr, Carter PA. Evolutionary physiology. Annu Rev Physiol. 1994;56:579–621. doi: 10.1146/annurev.ph.56.030194.003051. [DOI] [PubMed] [Google Scholar]
- 24.Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 25.Hochachka PW, Somero GN. Biochemical Adaptation: Mechanism and Process in Physiological Evolution. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 26.Garland T, Jr, Ives AR. Using the past to predict the present: Confidence intervals for regression equations in phylogenetic comparative methods. Am Nat. 2000;155:346–364. doi: 10.1086/303327. [DOI] [PubMed] [Google Scholar]
- 27.Garland T, Jr, Midford PE, Ives AR. An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. Am Zool. 1999;39:374–388. [Google Scholar]
- 28.Brashares J, Garland T, Jr, Arcese P. Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav Ecol. 2000;11:452–463. [Google Scholar]
- 29.Duncan RP, Forsyth DM, Hone J. Testing the metabolic theory of ecology: Allometric scaling exponents in mammals. Ecology. 2007;88:324–333. doi: 10.1890/0012-9658(2007)88[324:ttmtoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T., Jr Morphometrics of the avian small intestine, compared with nonflying mammals: A phylogenetic approach. Physiol Biochem Zool. 2008 doi: 10.1086/590395. in press. [DOI] [PubMed] [Google Scholar]
- 31.Cummins KW, Klug MJ. Feeding ecology of stream invertebrates. Annu Rev Ecol System. 1979;10:147–172. [Google Scholar]
- 32.Cutshall N. Turnover of zinc-65 in oysters. Health Phys. 1974;26:327–331. doi: 10.1097/00004032-197404000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Cain DJ, Luoma SN, Wallace WG. Linking metal bioaccumulation of aquatic insects to their distribution patterns in a mining-impacted river. Environ Toxicol Chem. 2004;23:1463–1473. doi: 10.1897/03-291. [DOI] [PubMed] [Google Scholar]
- 34.Clements WH. Community responses of stream organisms to heavy metals: A review of observational and experimental approaches. In: Newman MC, McIntosh AW, editors. Metal Ecotoxicology Concepts and Applications. Chelsea, MI: Lewis; 1991. pp. 363–386. [Google Scholar]
- 35.Evans DR, Balch GC, Evans HE, Welbourn PM. Simultaneous measurement of uptake and elimination of cadmium by caddisfly (Trichoptera:Hydropsychidae) larvae using stable isotope tracers. Environ Toxicol Chem. 2002;21:1032–1039. [PubMed] [Google Scholar]
- 36.Ritterhoff J, Zauke G-P. Potential role of metal-binding proteins in cadmium detoxification in Themisto libellula (Mandt) and Themisto abyssorum Boeck from the Greenland Sea. Mar Environ Res. 1998;45:179–191. [Google Scholar]
- 37.Suzuki KT, Sunaga H, Hatakeyama S, Sumi Y, Suzuki T. Differential binding of cadmium and copper to the same protein in a heavy metal tolerant species of mayfly (Baetis thermicus) larvae. Comp Biochem Physiol. 1989;94C:99–103. [Google Scholar]
- 38.Statzner B, Doledec S, Hugueny B. Biological trait composition of European stream invertebrate communities: Assessing the effects of various trait filter types. Ecography. 2004;27:470–488. [Google Scholar]
- 39.Clements WH. Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecol Appl. 2004;14:954–967. [Google Scholar]
- 40.Clark JL, Clements WH. The use of in situ and stream microcosm experiments to assess population- and community-level responses to metals. Environ Toxicol Chem. 2006;25:2306–2312. doi: 10.1897/05-552.1. [DOI] [PubMed] [Google Scholar]
- 41.Clements WH, Carlisle DM, Courtney LA, Harrahy EA. Integrating observational and experimental approaches to demonstrate causation in stream biomonitoring studies. Environ Toxicol Chem. 2002;21:1138–1146. [PubMed] [Google Scholar]
- 42.Garland T, Jr, Adolph SC. Physiological differentiation of vertebrate populations. Annu Rev Ecol Syst. 1991;22:193–228. [Google Scholar]
- 43.Rainbow PS. Accumulation of Zn, Cu, and Cd by crabs and barnacles. Estuarine Coastal Shelf Sci. 1985;21:669–686. [Google Scholar]
- 44.Rainbow PS, White SL. Comparative strategies of heavy metal accumulation by crustaceans: Zinc, copper, and cadmium in a decapod, amphipod, and a barnacle. Hydrobiologia. 1989;174:245–262. [Google Scholar]
- 45.Croteau M-N, Hare L, Tessier A. Difficulties in relating Cd concentrations in the predatory insect Chaoborus to those of its prey in nature. Can J Fish Aquat Sci. 2003;60:800–808. [Google Scholar]
- 46.Munger C, Hare L, Tessier A. Cadmium sources and exchange rates for Chaoborus larvae in nature. Limnol Oceanogr. 1999;44:1763–1771. [Google Scholar]
- 47.Garland T, Jr, Bennett AF, Rezende EL. Phylogenetic approaches in comparative physiology. J Exp Biol. 2005;208:3015–3035. doi: 10.1242/jeb.01745. [DOI] [PubMed] [Google Scholar]
- 48.Croteau M-N, Luoma SN, Topping BR, Lopez CB. Stable metal isotopes reveal copper accumulation and loss dynamics in the freshwater bivalve Corbicula. Environ Sci Technol. 2004;38:5002–5009. doi: 10.1021/es049432q. [DOI] [PubMed] [Google Scholar]
- 49.Wallace WG, Lopez GR. Bioavailability of biologically sequestered cadmium and the implications of metal toxicity. Mar Ecol Prog Ser. 1997;147:149–157. [Google Scholar]
- 50.Suzuki KT, et al. Binding of cadmium and copper in the mayfly Baetis thermicus larvae that inhabit a river polluted with heavy metals. Comp Biochem Physiol. 1988;19C:487–492. [Google Scholar]
- 51.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.