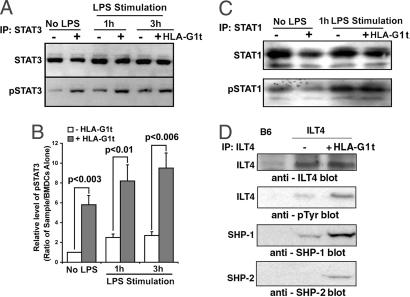
Fig. 3.
Engagement of ILT4 receptors by HLA-G1t increased STAT3 activation, phosphorylation of ILT4, and recruitment of SHP-1 and SHP-2. (A and B) Levels of STAT3 activation in ILT4-positive BMDCs treated with HLA-G1t for 3 h with and without stimulation with 100 ng/ml LPS for the indicated time. ILT4-positive BMDCs (5 × 106) were treated for 3 h with 50 ng/ml HLA-G1t or were left untreated. DCs were stimulated for an additional 1 h with 100 ng/ml LPS or left unstimulated. Cells were lysed, and proteins were immunoprecipitated (IP) with anti-STAT3 or anti-STAT1 antibodies. Immunoprecipitates were analyzed on Western blot for STAT proteins (A, Upper) and reprobed with anti-phosphotyrosine antibody 4G10 (A, Lower). Proteins were visualized by enhanced chemiluminescence. (C) Levels of STAT1 activation in ILT4-positive BMDCs treated with HLA-G1t for 3 h with and without stimulation with 100 ng/ml LPS. (D) Phosphorylated ILT4 is associated with SHP-1 and SHP-2. BMDCs were generated from ILT4 transgenic and B6 (control) mice, and the receptors were immunoprecipitated from cell extracts by using an anti-ILT4 mAb (42D1). Immunoblotting was performed by using anti-ILT4 mAb or 4G10, an anti-phosphotyrosine antibody as indicated. Immunoblots were reprobed sequentially with anti-SHP-1 and anti-SHP-2 antibodies as indicated. Proteins were visualized by enhanced chemiluminescence. Approximate molecular masses of the ILT4, SHP-1, and SHP-2 proteins were 95, 68, and 72 kDa, respectively. Data are representative of four independent experiments.