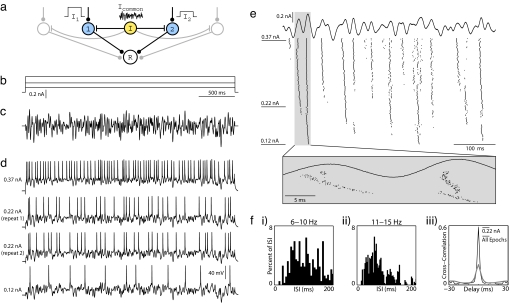
Fig. 1.
Protocol for studying synchrony induced by common noisy oscillations in vitro. (a) In our conceptual network model, a common noisy input (Icommon) modulates spike timing within a population of otherwise independently driven neurons (cells 1 and 2), and synchronous population activity is detected by a postsynaptic read-out neuron (R). (b and c) To reproduce this model experimentally, whole-cell patch recordings from layer 2/3 pyramidal neurons were stimulated in current clamp for 3 s with varying levels of constant current (b) combined with a common noisy oscillatory drive (c). The noisy waveform was generated to have a Gaussian Fourier spectrum, with a mean of 30 Hz and width of 30 Hz. Its rms amplitude was rescaled to 50% of the dynamic range of constant levels (Arms = 50%). (d) Four voltage traces from a set of 150 stimulus epochs on a single neuron are shown, including the minimum current (Lower) and the maximum current (Upper) injected, as well as two examples from an intermediate current. Step amplitudes are 0.12, 0.22, and 0.37 nA. (e) Fifty constant steps of uniform spacing were presented in random order, with each step repeated three times sequentially for a total of 150 noisy stimulus epochs. Step amplitudes were chosen to elicit firing between 0 and ≈30 Hz. Spike rasters are presented here for all 150 stimulus epochs during the first 500 ms of stimulation. Stimulus epochs are sorted by increasing stimulus amplitude from bottom to top. The expanded box illustrates changes in spike timing over fine time scales with increasing constant current. (f) (i–ii) Interspike interval distributions from a subset of stimulus epochs, pooled into successive 5-Hz ranges. Spike arrival times are irregular. (iii) Cross-correlograms obtained by comparing spike responses to 0.22-nA epochs only (black line) and all 150 epochs (gray line). Spikes were binned at 2 ms.