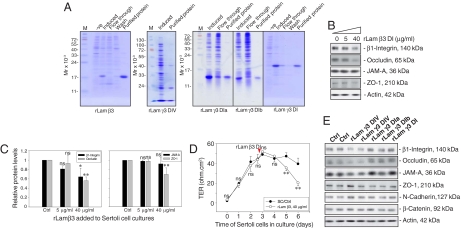
Fig. 1.
A study to assess the effects of different laminin fragments on the steady-state levels of proteins at the BTB and the BTB integrity in primary Sertoli cell cultures. (A) Purification of the recombinant proteins expressed in E. coli. Uninduced (-ve) and induced protein lysates from E. coli, fractions collected from the Ni column (both flow through and wash), and purified proteins were resolved by SDS-PAGE with gels stained by Coomassie blue. (B) The 5 and 40 μg/ml recombinant proteins corresponding to part of domain I of laminin β3 chain (rLam β3) were added to cultures on day 3 after isolation. Recombinant proteins were included in the daily replacement F12/DMEM for an additional 3 days. Cells were terminated on day 6 and lysates were used for immunoblotting, illustrating a dose-dependent inhibition on the production of β1-integrin, occludin, and ZO-1 but not JAM-A. Actin served as a loading control. (C) Immunoblot data were densitometrically scanned and compared. Each bar is the mean ± SD of n = 3, normalized against actin, wherein the control was arbitrarily set at 1, against which one-way ANOVA was performed. (D) Effects of the rLam β3 domain I on the TJ barrier. A total of 40 μg/ml rLam β3 domain I was added on day 2.5 (see red arrowhead), which was also included in the daily replacement F12/DMEM (n = 3). (E) Results of representative immunoblots illustrating the effects of other laminin fragments at 40 μg/ml on Sertoli cell target proteins using the experimental conditions of (B). Ctrl, control; rLam, recombinant laminin; DIV, domain IV; rLam γ3 DIa or DIb, recombinant laminin γ3 chain containing Domain Ia or Domain Ib (Table S1); DI, domain I; ns, not significantly different; ∗, P < 0.05; ∗∗, P < 0.01.