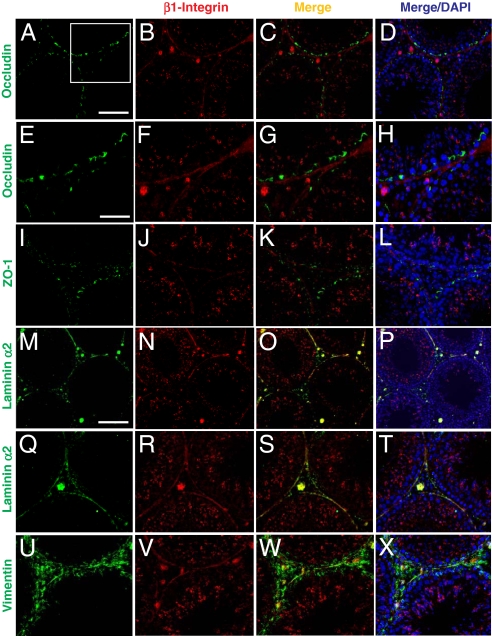
Fig. 3.
A study by immunofluoresent microscopy to examine the localization of β1-integrin in rat testes illustrating its presence at the apical ES and hemidesmosome. (A–D) Occludin (green, FITC) was localized at the BTB in the seminiferous epithelium of normal testes. β1-Integrin (red, Cy3) was predominantly localized at the apical ES, and it was also localized in the basal compartment of the epithelium. However, the signal of β1-integrin in the basal compartment was predominantly found underneath occludin in the merged image (C). (D) DAPI staining of nuclei. Boxed imaged in A was magnified in E–H, illustrating most β1-integrin was localized below the BTB (occludin) (see G and H vs. E and F). (I–L) ZO-1 (green, FITC) was also localized at the BTB above β1-integrin (red, Cy3). (M–P) Laminin α2 (green, FITC, a known basement membrane protein in rodent testes (19) was localized along the basement membrane of the seminiferous tubules as well as Leydig cells in the interstitium. It was shown to colocalize with β1-integrin in the basal compartment, as shown in the merged image (O, see yellowish color). (Q–T) Higher magnification showing the colocalization of β1-integrin with laminin α2 at the hemidesmosomes. (U–X) Vimentin (green, FITC; an intermediate filament structural protein), was localized at both basement membrane as well as at the BTB. β1-integrin (red, Cy3) was found to colocalize with vimentin at the basement membrane (see yellow color in W). (Scale bar in A: 100 μm, which applies to B–D, I–L, and Q–T; in M: 120 μm, which applies to N–P; in E: 50 μm, which applies to F–H and U–X.)