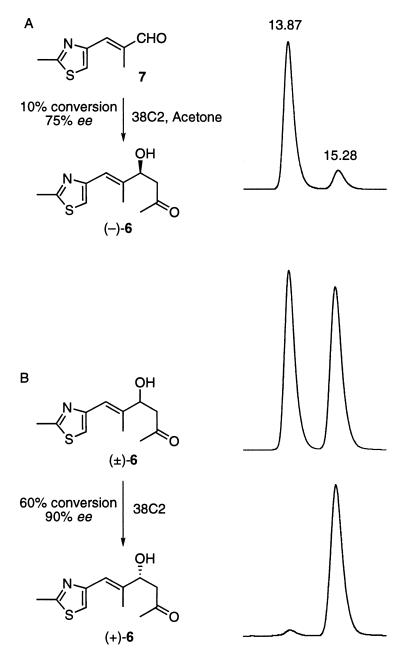
Figure 3.
(A) Antibody 38C2-catalyzed enantioselective aldol reaction of acetone with aldehyde 7. HPLC trace of (−)-6 (right). [HPLC conditions: λmax = 254 nm; Chiralcel OD-R (250 × 4.6 mm) column using 0.1% trifluoroacetic acid in acetonitrile/water (3:17) at a flow rate of 0.4 ml/min)]. A mixture of aldehyde 7 (1.28 g, 7.7 mmol) and antibody 38C2 (0.64 g, 0.0046 mmol) in PBS (total volume of 640 ml containing 64 ml of acetone) was stirred slowly at room temperature for 4 days. Antibody was filtered using Amicon membranes. Water solutions were passed through a reverse-phase (C18) column to elute water, and methanol was used as solvent to elute organics. Solvents were evaporated and separated over silica gel (hexane/ethyl acetate) to afford 0.11 g of pure (−)-6 and recovered 7 (1.12 g). (B) Antibody 38C2 catalyzed enantioselective retro-aldol reaction of (±)-6 (upper left) to (+)-6 (lower left). HPLC traces of (±)-6 (upper right) and reolved product (+)-6 (lower right). [HPLC conditions: Same as above.] Compound (±)-6 (1.32 g, 5.85 mmol in 50 ml of acetonitrile) was stirred slowly with antibody 38C2 (0.5 g, 0.00357 mmol) in PBS (pH 7.4) in a total volume of 500 ml. At >90% consumption of one enantiomer as judged by HPLC analysis (Right), the reaction mixture was filtered to recover antibodies and passed through a reverse-phase column as above to afford aldehyde 7 (0.44 g, 2.65 mmol) and (+)-6 (0.49g, 37%).