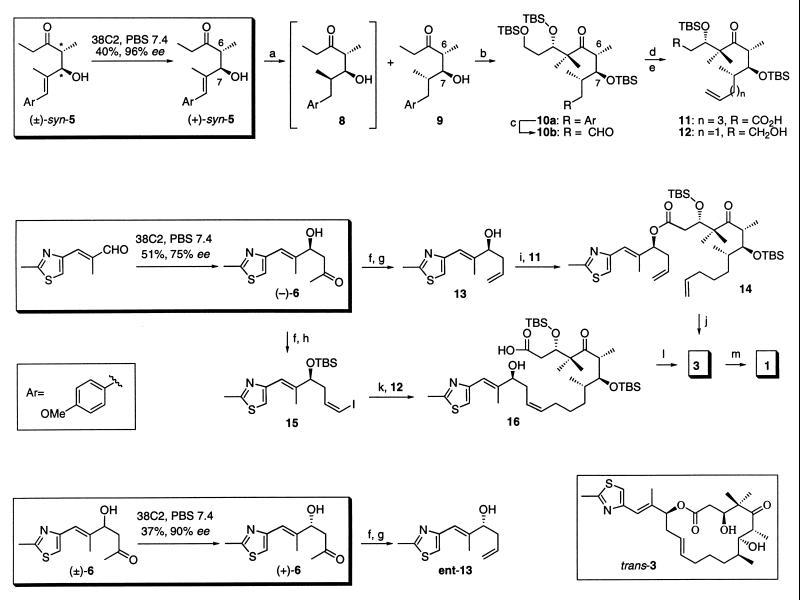
Scheme 2.
Total synthesis of epothilones A (1) and C (3) from (±)-syn-5 and aldehyde 7 via antibody-catalyzed retro-aldol and aldol reactions and synthesis of ent-13 from (±)-6 via antibody-catalyzed retro-aldol reaction. (a) H2, Rh/Al2O3, THF, 12 hr. (b) TBSCl, imidazole, DMF, 40–50°C, 48 hr; lithiumdiisopropylamide (LDA), THF, −78°C then MeI, hexamethylphosphoiamide (HMPA), −78°C to 0°C, 4 hr; LDA, THF, −78°C to −30°C, 2–5 hr; TBSOCH2CH2CHO, 0.5 hr (90%; 3S:3R, 2:1); TBSOTf, lutidine, CH2Cl2, −30°C, 3 hr, separation from 3-epi-10a by chromatotrone. (c) RuCl3, NaIO4, CCl4, CH3CN, PBS buffer (pH 7.4), 12 hr; CH2N2, MeOH–Et2O, 12 hr, 0°C; diisobutylaluminum hydride (DIBAL-H), THF, −78°C to −40°C, 2 hr; Dess–Martin reagent, CH2Cl2, 2 hr. (d) For the synthesis of 11: (EtO)2P(O)CH2CO2Et, NaH, THF, 0.5 hr; H2, Rh–Al2O3, THF, 12 hr; DIBAL-H, THF, −78°C to −40°C, 2 hr; Dess–Martin reagent, CH2Cl2, 2 hr; MePPh3I, BuLi, THF, 0°C, 0.5 hr; TsOH, CH2Cl2-MeOH, 0°C, 0.5 hr; Dess–Martin reagent, CH2Cl2, room temperature, 2 hr; NaClO2, tert-butylhydroperoxide (TBHP), 2-methyl-2-butene, NaH2PO4, THF, H2O, room temperature, 2 hr. (e) For the synthesis of 12: MePPh3I, BuLi, THF, 0°C, 0.5 hr; TsOH, CH2Cl2/MeOH, 0°C, 0.5 hr. (f) TBSCl, imidazole, DMF, room temperature, 16 hr; TMSOTf, lutidine, CH2Cl2, −78°C, 3 hr then excess m-CPBA, −78°C, room temperature, 2 hr; NaBH4, EtOH, 0°C, 0.5 hr; Pb(OAc)4, benzene, 0°C, 1 hr. (g) MePPh3I, BuLi, THF, 0°C, 0.5 hr; tetrabutylammonium fluoride (TBAF), THF, 0°C, 2 hr. (h) MePPh3I, BuLi, THF, 0°C, 0.5 hr, then I2, −78°C, 10 min, then NaHMDS, −20°C, 10 min, then aldehyde from (−)-6, 0.5 hr. (i) EDC, CH2Cl2, 0°C, room temperature, 12 hr. (j) Grubb’s catalyst, benzene, room temperature, 16 hr. (k) Reaction 1: compound 12, 9-borabicyclo[3:3:1]nonane (9-BBN), THF, 0°C, room temperature, 4 hr, reaction 2: compound 15, PdCl2(dppf)2, Cs2CO3, Ph3 As, H2O, DMF, room temperature, 1 hr, then reaction 2 added to reaction 1, room temperature, 4 hr; TBAF, THF, room temperature, 8 hr. (l) 2,4,6-Cl3C6H2COCl, Et3N, THF, 0°C, 0.5 hr; diluted with toluene and added dropwise to 4-dimethylaminipyridine (DMAP), toluene, room temperature, 2 hr. (m) Trifluoroacetic acid, CH2Cl2, −20 to 0°C, 2 hr; separation from its trans isomer using chromatotrone; solvent: CH2Cl2/MeOH, 49:1, (44% of 3 and 29% of trans isomer of 3, from 14; 40% of 3 and 19% of trans isomer of 3, from 16); CF3C(O)Me, oxone, acetonitrile, 0°C 1 hr, 55%.