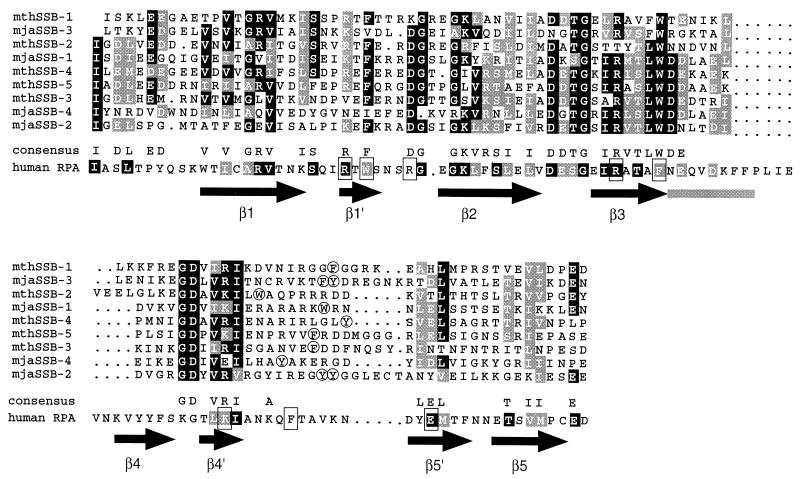
Figure 1.
Alignment of the sequences of the ssDNA-binding domains of M. jannaschii and M. thermoautotrophicum SSBs. The four repeats of mjaSSB and the five repeats of mthSSB are numbered sequentially. Identical residues are shaded black, and conserved residues are shaded gray. The sequence of the first ssDNA-binding domain of human RPA70 is shown below the consensus for the archaeal sequences. The shaded residues in the RPA sequence are similar or identical to those of the archaeal consensus. The boxed residues are those that make contact with the ssDNA in the crystal structure of hRPA70 (3). Arrows indicate the positions of the β sheets in the conserved OB (oligonucleotide/oligosaccharide binding) fold of hRPA70, and the gray bar indicates the position of an α helix. Aromatic residues corresponding to the conserved phenylalanine in the in the loop between sheets 4′ and 5′ of RPA70 are circled.