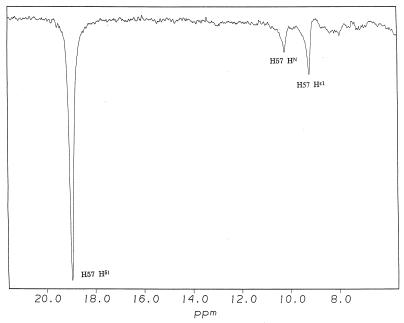
Figure 3.
Determination of the chemical shift of Hɛ1 of His57 in chymotrypsin-peptidyl-TFK complexes. Shown are representative truncated driven nuclear Overhauser effect difference spectra of low field 1H NMR signals in the complex of N-AcLF-CF3 and chymotrypsin. The 18.95-ppm peak is assigned to the proton bridging His 57 and Asp 102. This signal was saturated selectively for 25 ms. The chemical shift of Hɛ1 is assigned as labeled. The peak labeled H57HN is from the nearby backbone amide proton of His 57 as described (8).