Figure 1.
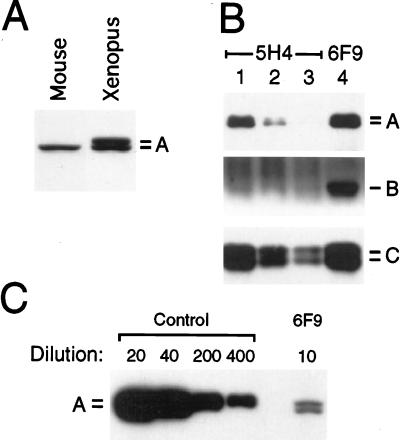
Quantitation of PP2A in Xenopus egg cytosol. (A) Equal amounts (5 μg) of protein from 10T1/2 cell cytoplasm and Xenopus egg cytosol were analyzed by Western blotting with mAb 6G3, which recognizes the A subunit of PP2A (30). Xenopus A subunit migrates as a doublet on SDS/PAGE as described (41). By using the amount of A subunit in 10T1/2 cell extracts (30) and a known amount of purified A subunit (data not shown) as a measure, we estimated that PP2A represents ≈1% of total protein in egg cytosol. (B) Egg cytosol was precipitated sequentially three times with mAb 5H4 (lanes 1–3), which binds to core enzyme (subunits A and C) but not to holoenzyme (subunits A, B, and C). After removing essentially all the core enzyme, a fourth precipitation was carried out with mAb 6F9 to isolate holoenzyme (lane 4). The result shows that holoenzyme is roughly as abundant in egg extracts as the core enzyme. Identification of all three subunits by Western blotting was performed as described (30). (C) PP2A was depleted from egg cytosol with mAb 6F9. To determine the degree of depletion, a serial dilution of undepleted egg cytosol was compared with depleted cytosol by Western blotting with mAb 6G3. A 10-fold dilution of depleted extract contained less A subunit than a 400-fold dilution of undepleted extract. Therefore, the depletion was >97.5%.