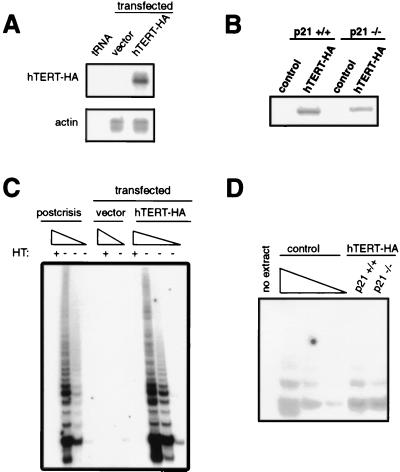
Figure 3.
hTERT-HA expression restores telomerase activity in postsenescent human cells. (A) Control or hTERT-HA expression vectors were stably introduced into a T-Ag-transformed HEK cell clone, yielding multiple clonal populations. Total RNA (40 μg) was isolated and assayed for hTERT-HA expression by an RNase protection assay using an antisense hTERT probe that recognizes and distinguishes endogenous hTERT and retroviral hTERT-HA transcripts. Results from a representative control and a high hTERT-HA-expressing clone are shown. Yeast tRNA and a human β-actin probe demonstrate the specificity of the probe and the presence of equal amounts of RNA, respectively. (B) Control or hTERT-HA expression vectors were stably introduced into p21+/+ and p21−/− fibroblasts, yielding multiple clonal populations. Representative clones from each infection are shown. Total cellular extracts were immunoblotted with an anti-HA antibody to visualize hTERT-HA. (C) Cytosolic cellular extracts prepared from the same control (0.2 μg) and hTERT-HA-transfected HEK clones (2, 0.2, or 0.02 μg) or from the parental line postcrisis were assayed for telomerase activity. As a negative control, 2 μg of all the extracts tested was heat treated (HT) to inactivate telomerase before telomere repeat amplification protocol assay. (D) Four micrograms of cytosolic cellular extracts prepared from p21+/+ or p21−/− fibroblast clones was assayed for telomerase activity. Telomerase-positive HeLa cell cytosolic extracts (4, 2, and 1 μg) are included as a positive control.