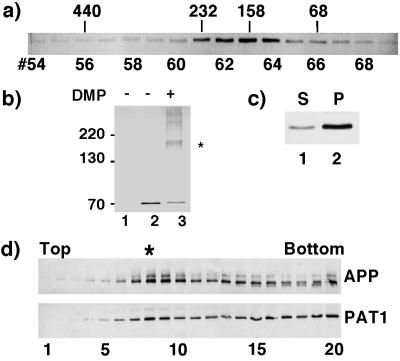
Figure 3.
PAT1 exists as an oligomeric complex, associates with the membrane and cofractionates with APP. (a) Gel filtration: Cytosol prepared from COS-1 cells transiently transfected with PAT1 in pFLAG-CMV-2 vector was separated on a Superose 12 gel filtration column. The fractions were Western blotted and probed with anti-FLAG M5 antibody and detected by alkaline phosphatase reaction. The elution positions of protein standards (in kDa) are indicated. (b) Chemical cross-linking: COS-1 cells mock-transfected (lane 1) or transiently transfected with FLAG-PAT1 in (lanes 2 and 3) were treated without (lane 2) or with (lane 3) 10 mM dimethyl pimelimidate (DMP, Pierce) at 4°C for 60 min. After the reaction was quenched, the cells were directly lysed in SDS/PAGE sample buffer and analyzed by SDS/PAGE and Western blotting as above. ∗ indicates the cross-linked band of 160–180 kDa detected by M5 antibody. The additional bands may be higher oligomers. (c) Subcellular fractionation: FLAG-PAT1 transfected COS-1 cells were homogenized and centrifuged at 150,000 × g (TLA 120.2, Beckman), and the supernatant (lane 1) and the pellet (lane 2) fractions were analyzed by SDS/PAGE and Western blotting. Proportion of PAT1 in pellet fraction varied between 50% and 75%. (d) Cofractionation of PAT1 and APP. A postnuclear supernatant from COS-1 cells transfected with FLAG-PAT1 and APP was fractionated by sucrose gradient centrifugation, and the fractions were analyzed by SDS/PAGE and Western blotting and probed with anti-APP (22C11; upper panel) or anti-FLAG antibody (lower panel). Note that a portion of PAT1 cofractionates with APP (fraction 8, indicated by ∗). The quantitation of these data is presented on the PNAS web site (www.pnas.org) as Fig. 3e.