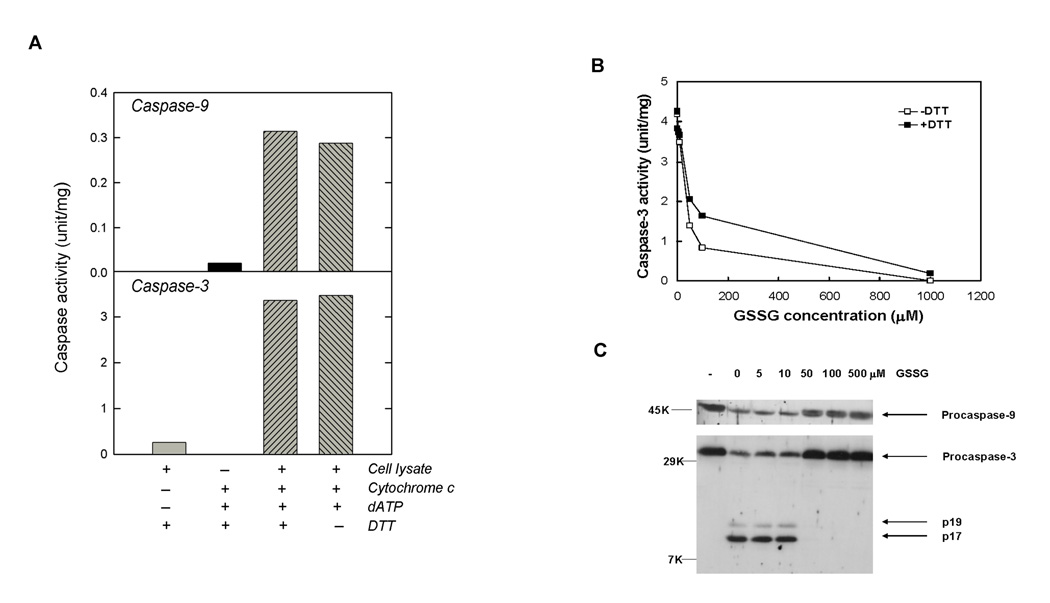
Figure 2.
Inhibition of Caspase-9 and Caspase-3 by GSSG in a Cell-Free System. (A) Activation of Caspase-9 and Caspase-3 in HL-60 Cytosolic Cell Lysates by Cytochrome c and dATP. HL-60 cytosolic cell lysates (100 µg protein) were incubated with +/− 10 µM cytochrome c, +/− 1 mM dATP and +/− 1 mM DTT for 60 min at 37°C. Reactions were stopped by addition of caspase activity buffer. Activities of caspase-9 and caspase-3 were measured using synthetic substrates Ac-LEHD-pNA and Ac-DEVD-pNA, respectively. (B) Inhibition of caspase-3 activation by GSSG. HL-60 cytosolic cell lysates (100 µg protein) were preincubated with different concentrations of GSSG for 90 min at room temperature. Cytochrome c and dATP were added to activate caspases. After 60 min at 37°C, reactions were stopped by addition of caspase activity buffer. Caspase-3 activity was measured in the presence (solid square) or absence of 5 mM DTT (open square) using the synthetic tetrapeptide substrate. (C) Inhibition of caspase cleavage by GSSG. Resulting reaction solutions (100 µl) after caspase activation (above) were directly subjected to western blot analysis for caspase-3 and caspase-9 using rabbit anti-caspase-3 and anti-caspase-9, respectively. Lane 1 (labeled “-“) contained no cytochrome C and dATP.