Abstract
Infection with many encephalitic viruses is associated with the induction of the proinflammatory cytokine interleukin (IL)-6. In some situations, induction of high levels of this cytokine is associated with a protective response, but in others it can be linked to tissue damage and disease. In the studies reported here, levels of serum IL-6 and virus-specific antibodies were measured on admission to hospital and correlated with clinical outcomes. Only some patients demonstrated raised levels of serum IL-6, and there was no correlation between high levels of this cytokine and either gender or the severity of clinical disease. A statistically significant association between raised IL-6 and age was observed, with all individuals below the age of 26 showing normal levels of serum IL-6, regardless of clinical presentation. Furthermore, not all patients had detectable levels of virus-specific serum immunoglobulin G (IgG) antibodies, but an inverse and statistically significant correlation between raised IL-6 levels and IgG titre was observed. Consequently, serum levels of IL-6 cannot be used as a reliable indicator of disease outcome.
Keywords: antibody, CNS, cytokines, encephalitis, flavivirus, IL-6, immunity, tick-borne encephalitis
Introduction
Tick-borne encephalitis (TBE) is a severe acute disease caused by a virus which belongs to the Flaviviridae family [1]. The main clinical symptoms of the disease are fever, headache, giddiness and asthenia, which sometimes lead to severe complications such as meningitis, meningoencephalitis or meningomyeloencephalitis. Currently, all hospitalized patients diagnosed with TBE are treated under intensive nursing protocols to control fever, maintain homeostasis and minimize rises in intracranial pressure. This process has been shown to improve clinical outcome, but places a significant burden on local services. If, however, patients who would develop only fever can be identified, they can be treated with commonly available cox 2 inhibitors and other anti-pyretics.
As is the case with most acute viral diseases, TBE infection is accompanied generally by cytokine production and specific antibody induction. Interleukin (IL)-6 is a proinflammatory multi-functional cytokine which plays a key role in maturation of antibody-producing B cells [2] and its role in the control and pathogenesis of several viral diseases has been reported [3]. Cytokine production has been shown to be triggered by several virus components, including surface glycoproteins, double-stranded RNA and intracellular viral proteins. These viral components have been shown to activate the major proinflammatory signalling pathways which lead to the production of cytokines and chemokines, with the transcription nuclear factor (NF) kappa B playing a prominent role. Activation of these signalling pathways can be induced simply by virus binding to the cell surface or as the result of more profound disruption of cellular metabolism, such as overloading of the cell's protein synthesis machinery. Raised levels of serum IL-6 have been shown to be induced in primary herpes simplex virus (HSV) infection [4], cytomegalovirus infections [5], acute Epstein–Barr virus infections [6], influenza [7], viral hepatitis [8], acute human immunodeficiency virus (HIV) infections [9] and diseases associated with human T cell leukaemia virus [10].
In most cases, IL-6 production is associated with a protective immune response, but in some cases it has been implicated as a cause of disease. Liver damage observed during acute or chronic hepatitis B virus-induced hepatitis appears to be caused by intrahepatic inflammatory processes, and excessive synthesis of IL-6 has been reported to lead to liver cirrhosis and hepatocellular carcinoma [8]. An imbalance in the production of several inflammatory cytokines, including IL-6, has been implicated in severe forms of influenza [11]. The role of IL-6 in both the pathogenesis and the control of virus-induced central nervous system (CNS) disease has been described on several occasions. Reactivation of HSV infection in neuronal cells has been reported to be caused by IL-6 inducing changes in the levels of the cellular transcription factors NF-IL-6 and signal transducer and activator of transcription 3 [12]. Studies with lymphocytic choriomeningitis virus and vesicular stomatitis virus suggest that IL-6 produced in both microglial cells and astrocytes may contribute to tissue repair mechanisms in the CNS by secretion of nerve growth factors [3]. Studies on simian immunodeficiency virus (SIV) infection in non-human primates have revealed further evidence for the role of IL-6 in virus-induced CNS disease [13,14]. Research on other retroviruses have suggested a molecular mechanism whereby the transcription of several host genes, including those involved in the IL-6 pathways, can be up-regulated through transactivation by the viral Tat gene [15]. Increased synthesis of IL-6 in the brain probably serves to protect the brain, but unregulated production can cause harm, probably through glial activation in severe inflammatory lesions. A role for IL-6 in the pathology of another lentiviral disease, feline immune deficiency virus, has also been reported [16,17]. In the study reported here, the role of IL-6 in TBE was investigated by examining the levels of serum IL-6 and virus-specific antibodies in hospitalized TBE patients, and matching them to the patient's age and the severity of clinical CNS disease.
Materials and methods
Selection of patients
Patients aged 15–80 years, hospitalized in the Eikatherinburg City Centre of Endemic Infections (Ural Region of Russia), and who reported flu-like symptoms (headache, giddiness and asthenia) 5–7 days previously, and a recent tick bite by anamnesis were recruited into the study. This area of study was chosen as TBE has been endemic in this region for several decades and 300–500 cases are reported every year. Care was taken to ensure that these patients had no history of active or passive specific prophylaxis for TBE, as vaccination or the administration of human immunoglobulin (Ig) is common for most visitors and TBE patients in many regions of Russia where TBE is endemic. The study was performed for 2 years, which included four TBE epidemic seasons in 2003–04, with samples being taken for diagnostic purposes during the first 24 h of hospitalization. TBE diagnosis was based on clinical presentation, a positive IgM result (according to the manufacturer's criteria) in a TBE-specific enzyme-linked immunosorbent assay (ELISA) and an increase in virus-specific IgG titres in samples taken at two or more time-points after admission. All patients in this study had an elevated temperature for as least 5 days; symptoms of meningitis, meningoencephalitis or meningomyeloencephalitis have been described previously [18].
No additional samples were taken for this investigation and all human materials studied were residues from samples taken for the routine diagnosis and management of these patients. Ethical approval for this study was obtained from the Clinical Ethical Review Committee of the London School of Hygiene and Tropical Medicine.
Laboratory analysis of serum samples
Blood was obtained by venepuncture within 24 h of admission and serum obtained and stored at −20°C for less than 6 months before analysis. TBE-specific IgM and IgG antibodies were measured using commercial kits from Vector-Best Ltd (Novosibirsk Region, Russia), containing recombinant virus E protein as antigen. Measurement of serum IL-6 levels was carried out using Quantikine human IL-6 kits from R&D Systems (Minneapolis, USA); the limit of detection for this analysis was 6 pg/ml. All laboratory analyses were performed in triplicate and the data presented as an average of three values falling within the 90% confidence interval.
Statistical analysis
Statistical analyses were carried out using Student's t-test. A value of 1·7 for tcritical was assumed for 90% confidence limits of statistically significant correlations.
Results
During the period of investigation, from 2003 to 2004, which covered four epidemic seasons, 74 patients presenting with symptoms of TBE were studied. Of these, 52 developed meningitis, meningoencephalitis or meningomyeloencephalitis (CNS disease) and 22 fever only (Table 1). Levels of serum IL-6 in individuals varied widely and many patients had no detectable cytokine upon hospital admission. However, no significant differences in the mean IL-6 levels were found between the patient cohorts presenting with fever or developing CNS disease, or between the patient groups studied in 2003 or 2004 (Table 1 and Fig. 1). Similarly, there were no measurable differences in IL-6 levels between males and females in any group (data not shown). Four of the patients with CNS disease died, but neither mortality nor the persistence of clinical symptoms was related to either serum IL-6 levels or IgG titre (data not shown). The data presented here also suggest that there is little or no IL-6 response in the young or the very old presenting with febrile disease, but the statistical significance of this correlation is low. There was no overall correlation between age and serum IL-6 levels in patients developing CNS disease. However, if all patients are grouped as follows − adolescents and young adults (15–25 years), middle-aged (26–60 years) the elderly (61–81 years) − a significant difference is seen in IL-6 levels between the first group, among whom IL-6 has not been detected, and the other two groups (Fig. 2). Individuals without clinical illness do not have detectable levels of IL-6 in their sera [19].
Table 1.
Interleukin (IL)-6 levels in serum samples from tick-borne encephalitis patients.
| Clinical presentation | Total no. of patients studied | % IL-6 positive | IL-6 mean serum concentration among positives ± s.d. (pg/ml)* |
|---|---|---|---|
| Period of observation: 2003 | |||
| Fever | 9 | 44 | 19·3 ± 15·5 |
| CNS disease | 29 | 59 | 23·0 ± 17·3 |
| Period of observation: 2004 | |||
| Fever | 13 | 23 | 10·5 ± 5·3 |
| CNS disease | 23 | 30 | 12·5 ± 5·3 |
Limit of detection was 6 pg/ml. CNS, central nervous system; s.d., standard deviation.
Fig. 1.
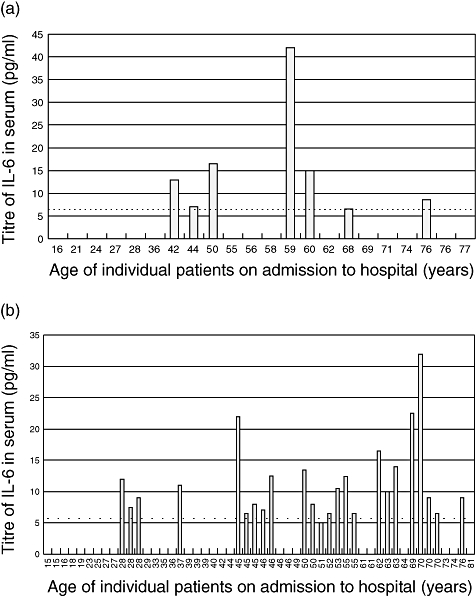
Relationship between serum interleukin (IL)-6 titres and age in patients with tick-borne encephalitis. Patients were classified by their age at the time of admission to hospital and IL-6 titres were measured as described in Methods. The limit of detection is shown by the dotted line. (a) Patients developing fever only. (b) Patients developing central nervous system disease.
Fig. 2.
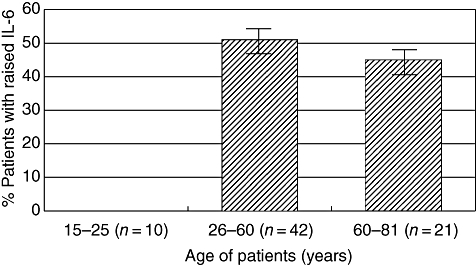
Serum interleukin (IL)-6 titres in patients stratified according to age.
Tick-borne encephalitis virus-specific IgG levels were also measured in serum from these patients (Fig. 3). Several of those with a febrile presentation had no detectable virus-specific IgG, but overall patients categorized as adolescents, young adults and middle-aged had higher levels of specific IgG than those classified as elderly. A similar pattern was seen in patients developing CNS disease, although overall the levels were lower than those seen in patients with fever. No correlation between IL-6 levels and IgG titres was seen in febrile patients, but those with CNS disease and high IgG titres had poor IL-6 responses and vice versa. Upon statistical analysis, however, none of the above observations on IgG titres proved to be statistically significant. The correlation between IL-6 concentration in all positive patients and the level of IgG antibodies was then analysed. Three groups of IL-6 positive patients with the IgG titres of 0 (group 1, n = 16), 1:100–1:1600 (group 2, n = 8) and 1:3200–1:6400 (group 3, n = 6) were selected. The average IL-6 concentration in the three groups was 11·9 pg/ml for group 1, 14·6 pg/ml for group 2 and 7·3 pg/ml for group3. Formal statistical analysis indicated that the difference between groups 1 and 2 was not statistically significant (t = 0·8), nor was the difference between groups 2 and 3 (t = 1·5). However, the difference between groups 1 and 3 was statistically significant (t = 2·1).
Fig. 3.
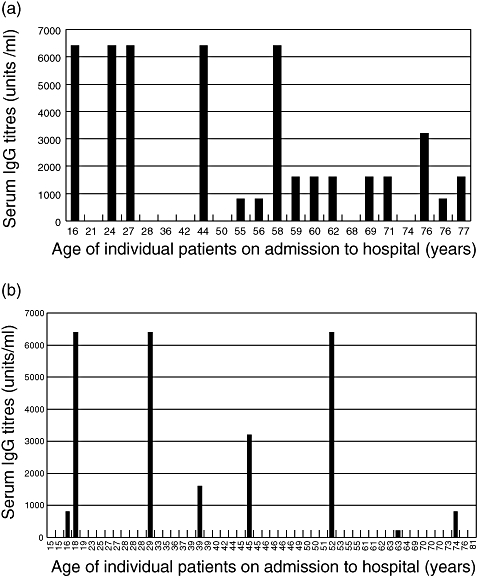
Relationship between serum immunoglobulin (IgG) and age in patients with tick-borne encephalitis. Patients were classified by their age at the time of admission to hospital and IgG titres were measured as described in Methods. (a) Patients developing fever only. (b) Patients developing central nervous system disease.
Discussion
Infection with TBE virus causes acute febrile illness in humans and can also give rise to potential fatal meningitis, meningoencephalitis or meningomyeloencephalitis. The number of hospitalized cases is rising in central and eastern Europe and also throughout Russia, despite the availability of safe and effective vaccines [20]. Intensive nursing protocols are applied currently to all hospitalized TBE patients to improve clinical outcome, but if those cases developing only fever can be identified they can be treated with anti-pyretics alone. Although neuroinvasion of TBE virus in humans has been well documented, the factors which convert an acute, non-fatal febrile infection into a severe and potentially fatal CNS disease are not known. The unregulated induction of proinflammatory cytokines has been described for several virus diseases, and a potential role for IL-6 in CNS tissue damage has been proposed. A possible mechanism for this deregulation of IL-6 production is the transactivation of cytokine genes by a viral protein and there is some evidence for this hypothesis from SIV infections of non-human primates [13,14]. In addition, recent data from mice infected experimentally with TBE virus have shown high levels of serum IL-6 in animals with encephalitis (A. V. Timofeev, unpublished data). Moreover, recent work by Park and colleagues [21] has suggested that tissue inflammation is regulated by a distinct lineage of IL-17-producing CD4 T cells, which act in concert with other cytokines such as IL-6 to produce their effect.
The studies reported here were designed to determine whether high levels of serum IL-6 determined at the moment of hospitalization of the TBE patients were associated with future severe CNS disease. If such a correlation could be demonstrated, it would aid the development of non-invasive diagnostic protocols and enable more appropriate clinical care and reduce the burden on local clinical services. In the cohorts investigated, however, only some patients, with either febrile or CNS disease, demonstrated raised levels of serum IL-6 and there was no correlation with high levels of this cytokine and disease severity. Interpretation of serum IL-6 levels as an indicator of CNS disease is difficult, as cytokines are released from systemic sources as well as the CNS. These results appear to be at variance with our earlier studies, which showed raised IL-6 levels in sera from patients with meningoencephalitis [19]. However, the number of patients included in these earlier studies were very small and the results were not statistically significant. Atrasheuskaya et al. [22] also found high levels of IL-6 in sera of recently hospitalized TBE patients with meningoencephalitis. These patients had, however, received donor-specific Ig a few days before hospitalization and interpretation of these studies is difficult, as antibodies and cytokines induced by virus infection could not be distinguished from those potentially introduced through donor Ig preparations. The study reported here recruited larger numbers of patients and ensured that they had not received any TBE-specific prophylaxis or therapy before admission.
A positive, statistically significant association between raised IL-6 and age was observed, with all individuals below the age of 26 showing normal levels of serum IL-6, regardless of clinical presentation. This observation is not accounted for easily by host genetic factors or virus genotype. Moreover, age-related susceptibility to disease arising from deficiencies in the immune system is normally reported in the very young and the elderly [23]. Environmental or cultural behaviours, leading to underlying subclinical conditions, may be responsible, but no evidence is currently available to support this conjecture.
An inverse and statistically significant correlation between raised IL-6 levels and IgG titre was also observed. This would suggest that despite the presence of high levels of a cytokine (IL-6) that should stimulate antibody production, other factors are suppressing it, suggesting that other unknown factors must be associated with raised IL-6 levels and severe CNS disease. Several factors could influence IL-6 synthesis in TBE, including virus genotype, the genetic background of the patient, pre-existing immunity to other flavivirus infections and co-infection with other tick-borne microorganisms. Little information is available on co-factors influencing IL-6 production in viral disease, but some studies on patient cohorts have suggested that susceptibility to HIV infection is associated with the haplotype of several cytokine genes, including IL-6 [24]. Other studies by Price et al. [25] have suggested that the susceptibility of HIV-infected individuals to other infections is also associated with the haplotype of some cytokine genes. Although the predisposition of infected individuals to develop CNS disease is not understood, it is likely to be the result of an interplay between several factors. As with HIV susceptibility to disease, caused possibly by an inherited inability to regulate cytokine production, could be important. Furthermore, the ability of different virus genotypes to induce IL-6 and other cytokines through the transactivation of host genes could also play a role. An additional potential issue to consider when discussing TBE is that the tick vector is known to carry a number of other microorganisms such as Borrelia, which are known to interfere with immune responses [26]. A further explanation for the variability in IgG induction is that the ELISA assay used detects only the virus E protein. However, there are no reports in the literature of any flavivirus virus infection or vaccination with a whole virus which induces antibodies to other virus-encoded proteins, to the exclusion of E protein. Further clinical studies are planned to test these hypotheses.
In conclusion, we have measured serum IL-6 and virus-specific antibodies in a cohort of patients admitted to hospital with suspected TBE, and developing either febrile illness of severe CNS disease. These patients had not received any immunotherapy prior to analysis. Raised titres of serum IL-6 were seen in patients with either form of disease, but not in all individuals within either group. A statistically significant correlation was seen between IL-6 levels and age, but not between IL-6 levels and disease severity. An inverse relationship between IL-6 levels and IgG titres was also seen, but again not between IgG levels and disease severity. These results suggest that raised serum IL-6 upon hospitalization cannot be used to predict future severity of diseases reliably, and other factors may be involved.
Acknowledgments
This work was supported by the Wellcome Trust, grant no. 064845.
References
- 1.Monath TP, Heinz FX. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, editors. Field's virology. 3. Philadelphia, PA: Lippincott and Raven; 1996. pp. 961–1034. [Google Scholar]
- 2.Kishimoto T. Interleukin-6: from basic science to medicine − 40 years in immunology. Annu Rev Immunol. 2003;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 3.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–94. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 4.Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–9. [PubMed] [Google Scholar]
- 5.Carlquist JF, Edelman L, Bennion DW, Anderson JL. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and a transcription factor, NF-κB. J Infect Dis. 1999;197:1094–100. doi: 10.1086/314734. [DOI] [PubMed] [Google Scholar]
- 6.Tanner JE, Alfieri C, Chatila TA, Diaz-Mitoma F. Induction of interleukin-6 after stimulation of human B-cell CD21 by Epstein–Barr virus glycoproteins gp350 and gp220. J Virol. 1996;70:570–5. doi: 10.1128/jvi.70.1.570-575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden FG, Fritz RS, Lobo MC, Alvord WG, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defence. J Clin Invest. 1998;101:643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Park US, Choi I, Yoon SK, Park YM, Lee YI. Human interleukin-6 gene is activated by hepatitis B virus-X protein in human hepatoma cells. Clin Cancer Res. 1998;4:1711–17. [PubMed] [Google Scholar]
- 9.Graziosi C, Gantt KR, Vaccarezza M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Aci Sci USA. 1996;93:4386–91. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori N, Shirakawa F, Abe M, et al. Human T-cell leukemia virus type 1 tat transactivates the interleukin-6 gene in human rheumatoid synovial cells. J Rheumatol. 1995;22:2049–954. [PubMed] [Google Scholar]
- 11.Lipatov AS, Andreansky S, Webby RJ, et al. Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortments in mice: the role of cytokines and B- and T-cell responses. J Gen Virol. 2005;86:1121–30. doi: 10.1099/vir.0.80663-0. [DOI] [PubMed] [Google Scholar]
- 12.Kriesel JD, Ricigliano J, Spruance SL, Garza HH, Hill JM. Neuronal activation of herpes simplex virus may involve interleukin 6. J Neurovirol. 1997;3:441–8. doi: 10.3109/13550289709031190. [DOI] [PubMed] [Google Scholar]
- 13.Roberts ES, Burudi EM, Flynn C, et al. Acute SIV infection of the brain leads to up regulation of IL-6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. J Neuroimmunol. 2004;157:81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Boche D, Khatissian E, Gray F, Falanga P, Montangier L, Hurtrel B. Viral load and neuropathology in the SIV model. J Neurovirol. 1999;5:232–40. doi: 10.3109/13550289909015809. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosino C, Ruocco MR, Chen X, et al. HIV-1 Tat induces the expression of the interleukin 6 (IL-6) gene by binding to the IL6 leader RNA and by interacting with CAAT enhancer binding protein beta (NF-IL6) J Biol Chem. 1997;272:14883–92. doi: 10.1074/jbc.272.23.14883. [DOI] [PubMed] [Google Scholar]
- 16.Ritchey JW, Levy JK, Bliss SK, Tompkins WA, Tompkins MB. Constitutive expression of types 1 and 2 cytokines by alveolar macrophages from feline immunodeficiency virus-infected cats. Vet Immunol Immunopathol. 2001;79:83–100. doi: 10.1016/S0165-2427(01)00250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery PR, Hoover EA. Gamma interferon/interleukin 10 balance in tissue lymphocytes correlates with down modulation of mucosal feline immunodeficiency virus infection. J Virol. 2004;78:4011–19. doi: 10.1128/JVI.78.8.4011-4019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorobyeva NN, Korenberg EI, Grigoryan YV. Diagnostics of tick-borne diseases in the endemic region of Russia. Wien Klin Wochenschr. 2003;114:610–12. [PubMed] [Google Scholar]
- 19.Timofeev AV, Kondratyeva YY, Orlovsky VG, Kuzhukov GP, Loktev VP, Karganova GG. Correlations between tick-borne encephalitis severity and concentration of interleukins 2 and 6 in the serum of patients with this disease. Ter Arkh. 2002;11:22–3. [Google Scholar]
- 20.Kunze U, Baumhackl U, Bretschneider R, et al. The golden agers and tick-borne encephalitis. Conference report and position paper of the International Scientific Working Group on Tick-borne encephalitis. International Scientific Working Group on Tick-borne encephalitis. Wien Med Wochenschr. 2005;155:289–94. doi: 10.1007/s10354-005-0178-0. [DOI] [PubMed] [Google Scholar]
- 21.Park H, Li Z, Yang XO, Chang SH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atrasheuskaya AV, Fredeking TM, Iganatyev GM. Changes in immune parameters and their correlation in human cases of tick-borne encephalitis. Clin Exptl Immunol. 2003;131:148–52. doi: 10.1046/j.1365-2249.2003.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burle A, Caselli G, Franceschi C, et al. Pathophysiology of ageing, longevity and age related diseases. Immun Ageing. 2007;4:4. doi: 10.1186/1742-4933-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Song W, Lobashevsky E, et al. Cytokine and chemokine gene polymorphisms among ethnically diverse North Americans with HIV-1 infections. J Acquir Immune Defic Syndr. 2004;15:446–54. doi: 10.1097/00126334-200404150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Price P, Morahan G, Huang D, et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–7. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 26.Kuenzle S, von Budingen H-C, Meier M, et al. Pathogen specificity and autoimmunity are distinct features of antigen-driven immune responses in neuroborreliosis. Infect Immun. 2007;75:3843–7. doi: 10.1128/IAI.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


