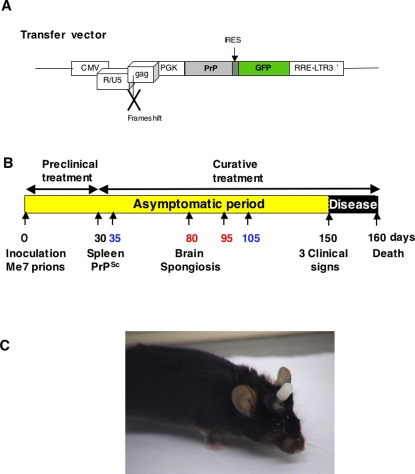
Figure 1.
Schematic representation of the transfer vector used for virions preparation (A). The transfer vector was derived from the prototypic FIV-14-Petaluma genome (AIDS Research and Reference Reagent Program) [28]. The U3 region of the 5′ LTR was replaced with CMV promotor in order to express the vector in the cells. The gag gene was truncated at position 1243 and a frameshift was introduced at position 926. The pol, vif, orfA, env and rev genes were replaced by a cassette containing the mutated PrPQ167R gene driven by the phosphoglycerate kinase promotor (PGK). The PrP gene is followed by an IRES sequence and enhanced GFP gene. As a control vector, we used a transfer vector containing only the GFP gene without the PrP sequence. Schema of the therapeutic protocol assayed in a mouse model of prion disease (B). C57Bl/6 mice inoculated with Me7 prion strain developed a prion disease detectable after 150 days post-infection (p.i.). The prion agent first replicates in the peripheral sites, especially the lymphoreticular system, before the neuroinvasion of the central nervous system (CNS). PrPSc is detectable in the spleen at 30 days p.i. and the spongiosis is visible in the brain at about 80 days p.i. [34]. Preclinical treatments (1–30 days) target the prion agent during its replication in the lymphoreticular system and before the neuroinvasion of the CNS. Curative treatments (30–160 days) target the infectious agent after the neuroinvasion of the CNS. To evaluate the possible curative effect of ΨPrPQ167R, several protocols were tested : (i) a unique injection of ΨPrPQ167R at 35 or 105 days p.i. (printed in blue); and (ii) two administrations of the ΨPrPQ167R at 80 and 95 days p.i. (printed in red). Picture of a C57Bl/6 mouse with a guide cannula implanted into its brain (C). An obturator has been fitted into the guide-cannula to prevent the channel from being blocked by tissue regeneration.