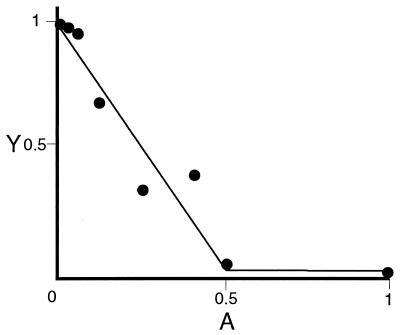
Figure 1.
Measurement of inhibitory activity of mAb with respect to specific P3A2–DNA complex formation. Relative occupancy of oligonucleotide probe (Y) is plotted against an antibody dilution series (A). The intercept, Y0 (here Y0 = 0.78 of total probe in the reaction) represents the probe occupancy in the absence of antibody (A = 0). The inhibitory activity is quantitatively estimated as the value of kA, the slope; here, kA = 1.26 × 1010 M−1. The data are fit to the function Y = αkA·A + Y0 (Eq. 1). Here Y is occupancy of oligonucleotide probe; i.e., where PD(A) dilution (antibody concentration was measured after purification of the antibody from the hybridoma ascites fluid) and D0 is concentration of total probe in the reaction: Y = PDA/D0. In Eq. 1, α = −2P0, where P0 is the molar quantity of P3A2 transcription factor in the reaction; in these experiments, P0 ranged from 2 to 5 × 10−11 M. kA is defined as the equilibrium constant for the reaction of a mAb molecule with two P3A2 molecules, i.e., kA = AP2/A · P02. Eq. (1) follows from the assumption that all P3A2 molecules will be in complex with either the antibody or the DNA probe and that the P3A2–DNA reaction is bimolecular, i.e., PD(A) = kDP(A) · D(A), where P(A) and D(A) are the molar concentrations of the free P3A2 protein and the DNA probe at given antibody dilutions.